一种水凝胶/P(VDF-TrFE)系统,通过可控的自维持电线索增强骨再生
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-29
DOI:10.1016/j.bioadv.2025.214482
引用次数: 0
摘要
临界尺寸骨缺损有限的自愈能力对其愈合提出了重大挑战。通过调节生物材料的理化性质来模拟自然骨再生微环境是一种有效的方法。在这项工作中,我们制备了基于壳聚糖-明胶(CS-Gel)的水凝胶/聚(聚氟乙烯-共三氟乙烯)(P(VDF-TrFE))系统,该系统为骨再生提供仿生和电线索。这些水凝胶膜构建在P(VDF-TrFE)层上,持续的电信号依赖于P(VDF-TrFE)的偶极子取向。这种双层结构将电刺激和生物相容性从化学成分中分离出来,允许在不改变水凝胶表面官能团的情况下产生自我维持的电信号。结果表明,这些水凝胶/P(VDF-TrFE)系统在体外更有效地极化巨噬细胞向替代活化(M2)表型发展,促进干细胞分化。此外,水凝胶/P(VDF-TrFE)系统增强抗炎细胞因子的表达,促进体内新骨形成。本研究提出了一种结合电线索和仿生环境来加速骨再生的有希望的策略,为具有自维持电线索的水凝胶/P(VDF-TrFE)系统的制备提供了一种新的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
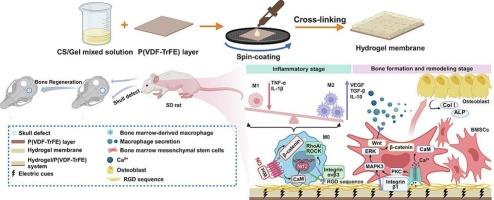
A hydrogel/P(VDF-TrFE) system for enhanced bone regeneration with controllable self-sustained electrical cues
The limited self-healing capacity of critical-sized bone defects presents significant challenges in healing. An effective approach is to regulate the physicochemical properties of biomaterials to mimic the natural bone regenerative microenvironment. In this work, we have prepared Chitosan-Gelatin (CS-Gel) based hydrogel/ Poly(vinylidene fluoride-co-trifluoroethylene) (P(VDF-TrFE)) systems, which provide biomimetic and electric cues for bone regeneration. These hydrogel membranes are constructed on P(VDF-TrFE) layers, and the sustained electrical cues rely on the dipole orientation of P(VDF-TrFE). The bilayer structure decouples electrical stimulation and biocompatibility from chemical composition, allowing the generation of self-sustained electrical cues without altering the functional groups on the hydrogel surface. The results show that these hydrogel/P(VDF-TrFE) systems are more effective in polarizing macrophages toward the alternatively activated (M2) phenotype and promoting stem cell differentiation in vitro. Furthermore, the hydrogel/P(VDF-TrFE) system enhances the expression of anti-inflammatory cytokines and encourages new bone formation in vivo. This work presents a promising strategy to accelerate bone regeneration by combining electrical cues and biomimetic environments, offering a new preparation method for hydrogel/P(VDF-TrFE) systems with self-sustained electrical cues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


