可调节胃内球囊治疗代谢功能障碍相关的脂肪变性肝病-增强体重减轻和组织学改善
IF 3.2
Q2 GASTROENTEROLOGY & HEPATOLOGY
Journal of Clinical and Experimental Hepatology
Pub Date : 2025-08-12
DOI:10.1016/j.jceh.2025.103152
引用次数: 0
摘要
背景/目的代谢功能障碍相关脂肪性肝病/代谢功能障碍相关脂肪性肝炎(MASLD/MASH)伴肥胖显著增加肝硬化的风险,除非体重显著减轻。这项前瞻性研究评估了可调节胃内球囊(aIGB)放置对MASLD/MASH代谢和组织学参数的影响,强调了其在实现疾病解决所必需的体重减轻方面的功效。方法36例患者(17例女性,平均年龄:39.8岁,平均体重指数:35.4 kg/m2)在基线和术后6个月(2020年9月至2023年2月)行aIGB放置并超声内镜引导下肝活检。排除活检不充分或早期球囊切除的患者。主要结局包括非酒精性脂肪性肝病活动评分(NAS)的变化;次要结局是体重减轻、谷丙转氨酶(ALT)、纤维化消退和不良事件(NCT04182646)。结果6个月平均总体重减轻(TBWL) 12.65%(95%可信区间:10.38 ~ 14.92),88.9%患者达到≥5% TBWL。25例(75%)患者需要调整球囊容量以解决减肥平台期或不耐受问题。ALT水平显著提高(86.36±27.14∶38.53±16.57;P < 0.001)。91.7%的患者NASs得到改善(中位数[四分位数间距]:3[3-4]至1[0-1.75]),55.56%的患者纤维化得到改善。球囊向上调整与NAS (P < 0.001)和脂肪变性(P = 0.002)的改善相关。无严重不良事件报告。结论igb可有效减轻MASLD/MASH患者的体重,改善其代谢和组织学参数,并可通过容积调节提高预后。该程序显示出极好的安全性。临床试验注册本研究注册号为NCT04182646。本文章由计算机程序翻译,如有差异,请以英文原文为准。
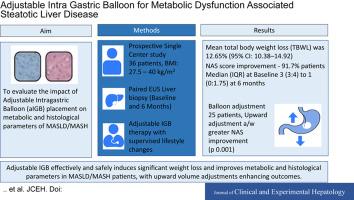
Adjustable Intragastric Balloon for Metabolic Dysfunction–associated Steatotic Liver Disease—Enhanced Weight Loss and Histological Improvement
Background/Aims
Metabolic dysfunction–associated steatotic liver disease/metabolic dysfunction–associated steatohepatitis (MASLD/MASH) with obesity significantly increases the risk of cirrhosis unless substantial weight loss is achieved. This prospective study evaluates the impact of adjustable intragastric balloon (aIGB) placement on metabolic and histological parameters of MASLD/MASH, highlighting its efficacy in achieving weight loss necessary for disease resolution.
Methods
Thirty-six patients (17 females; mean age: 39.8 years; mean body mass index: 35.4 kg/m2) underwent aIGB placement with endoscopic ultrasound–guided liver biopsy at baseline and six months post procedure (September 2020 to February 2023). Patients with inadequate biopsy or early balloon removal were excluded. Primary outcomes included changes in the non-alcoholic fatty liver disease Activity Score (NAS); secondary outcomes were weight loss, alanine transaminase (ALT), fibrosis regression, and adverse events (NCT04182646).
Results
At six months, the mean total body weight loss (TBWL) was 12.65% (95% confidence interval: 10.38-14.92), with ≥5% TBWL achieved in 88.9% of patients. Balloon volume adjustments were required in 25 patients (75%) to address weight-loss plateau or intolerance. ALT levels improved significantly (86.36 ± 27.14 vs. 38.53 ± 16.57; P < 0.001). NASs improved in 91.7% of patients (median [interquartile range]: 3 [3-4] to 1 [0-1.75]), and fibrosis improvement was observed in 55.56%. Upward balloon adjustments were associated with greater improvements in NAS (P < 0.001) and steatosis (P = 0.002). No serious adverse events were reported.
Conclusion
aIGB effectively induces significant weight loss and improves metabolic and histological parameters in MASLD/MASH patients, with upward volume adjustments enhancing outcomes. The procedure demonstrated an excellent safety profile.
Clinical trial registration
This study was registered under registration number NCT04182646.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Clinical and Experimental Hepatology
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
4.90
自引率
16.70%
发文量
537
审稿时长
64 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


