优化小囊泡型阿霉素脂质体配方,提高抗肿瘤效果
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
Talidox (TLD)是一种创新的阿霉素脂质体制剂,旨在提高药物传递效率,降低现有阿霉素脂质体制剂的全身毒性。该出版物整合了10年TLD的临床前研究,整合了物理化学表征,体外和体内疗效。与Caelyx相比,TLD具有更小的粒径和优化的药脂比,旨在提高肿瘤的穿透和摄取,从而提高治疗效果。乳腺癌和软组织肉瘤模型的临床前研究表明,TLD比游离阿霉素的疗效更好,并且具有非常好的安全性。这与已经公布的临床试验中发现的TLD有利的风险-收益比相辅相成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
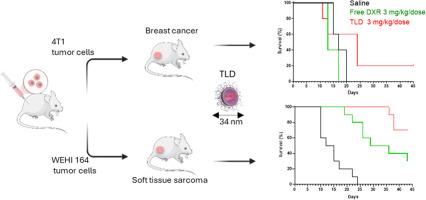
TLD: An optimized liposomal doxorubicin formulation with small vesicle size for improved anti-tumor efficacy
Talidox (TLD) is an innovative liposomal doxorubicin formulation designed to enhance drug delivery efficiency and reduce systemic toxicity over existing liposomal doxorubicin formulations. This publication consolidates 10 years of preclinical research on TLD, integrating physicochemical characterization, and in vitro and in vivo efficacy. TLD has a reduced particle size and optimized drug-to-lipid ratio compared to Caelyx, aiming to improve tumor penetration and uptake and therefore therapeutic efficacy. The preclinical studies in breast cancer and soft tissue sarcoma models highlight the improved efficacy of TLD over free doxorubicin combined with a very good safety profile. This is complemented by the already published favorable risk-benefit ratio of TLD found in clinical trials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


