吸入治疗药物的控制释放和延长肺保留:综述
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
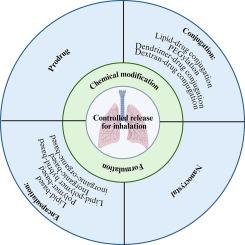
肺治疗药物控释系统的发展已成为制药研究的一个重要焦点。为了调节治疗剂的释放速度,人们采用了多种策略,既针对水溶性差的药物增强释放,又针对高水溶性化合物延长释放。这些努力主要集中在活性药物成分(api)的化学修饰和创新配方的创造,其中包括可生物降解的天然或合成赋形剂。本文综述了控释可吸入药物的进展,从肺部给药的概述开始。它解决了目前可吸入产品的分类,肺给药的优势,以及涉及肺内颗粒沉积的机制。本综述探讨了控释技术,主要分为两种主要方法:(i)活性药物成分(api)的化学修饰和(ii)专门配方。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Controlled release and extended pulmonary retention of inhaled therapeutics: A review
The advancement of controlled release systems for pulmonary therapeutic products has become a significant focus in pharmaceutical research. Various strategies have been employed to regulate the release rate of therapeutic agents, targeting both the release enhancement of poorly water-soluble drugs and prolonging the release of highly water-soluble compounds. These efforts primarily focus on chemical modifications of the active pharmaceutical ingredients (APIs) and the creation of innovative formulations that incorporate biodegradable natural or synthetic excipients. This review offers a comprehensive overview of advancements in controlled release inhalable drugs, beginning with the overview of pulmonary drug delivery. It addresses the classification of current inhalable products, the advantages of pulmonary administration, and the mechanisms involved in the particle deposition within the lungs. This review explores controlled release techniques, which are primarily divided into two main approaches: (i) chemical modification of active pharmaceutical ingredients (APIs) and (ii) specialized formulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


