n -碘苯甲酰吲哚光诱导苯酚有机催化合成异吲哚吲哚酮
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
建立了一种高效的光诱导苯酚有机催化合成异吲哚吲哚酮的方法,该方法具有底物范围广、产率高和良好的官能团耐受性。机理研究证实,受激发的酚酸盐催化剂促进了n -碘苯甲酰吲哚的直接单电子转移途径,并通过紫外可见光谱、荧光猝灭和循环伏安法进行了验证。这种方法可以合成具有重要生物学意义的衍生物,包括褪黑激素MT3和5-HT6受体配体。本文章由计算机程序翻译,如有差异,请以英文原文为准。

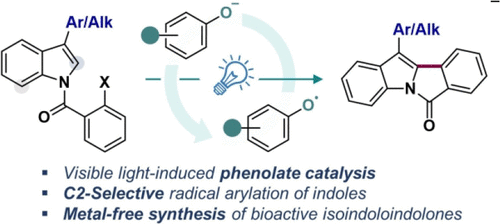
Photoinduced Phenolate Organocatalysis for Isoindoloindolone Synthesis with N-Iodobenzoyl Indoles
An efficient photoinduced phenolate organocatalytic methodology for the synthesis of isoindoloindolones was developed, demonstrating a broad substrate scope with high yields and excellent functional group tolerance. Mechanistic studies confirmed a direct single-electron transfer pathway facilitated by an excited phenolate catalyst to N-iodobenzoyl indoles, validated through UV–vis spectroscopy, fluorescence quenching, and cyclic voltammetry. This approach enables the synthesis of biologically significant derivatives including melatonin MT3 and 5-HT6 receptor ligands.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


