调整Fe三维自旋态,增强s掺杂magnetite@resorcinol-formaldehyde树脂核壳催化剂对原位生成H2O2的吸附/活化,促进级联自芬顿氧化
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
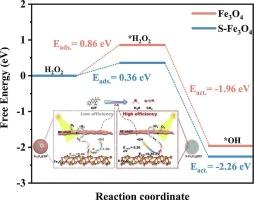
虽然光催化自fenton氧化技术通过原位生成H2O2实现了氧化剂的可持续利用,但由于H2O2在自fenton催化剂表面的低浓度和缓慢扩散,极大地限制了自fenton系统中有机污染物的氧化效率。本研究通过硫化策略调整铁基催化剂的电子结构,开发了一种核壳型硫掺杂磁铁矿(S-Fe3O4) @间苯二酚甲醛(RF)树脂光辅助自fenton催化剂。硫的引入促进了Fe(II)/Fe(III)的氧化还原循环,显著增强了对H2O2的吸附和活化,H2O2是由可见光负责的RF树脂薄壳原位生成的。该催化剂具有以上优点,实现了以环丙沙星(CIP)为代表的氟喹诺酮类抗生素的高效降解。自由基猝灭实验和电子自旋共振(ESR)分析表明,•OH和•O2−是S-Fe3O4@RF-mediated自fenton反应中的主要活性氧。系统表征和密度泛函理论(DFT)计算表明,硫化处理通过调节复合催化剂中铁元素的电子态密度,降低了H2O2生成•OH的活化能势垒,并阐明了自由基主导的污染物降解途径。催化剂独特的核壳结构不仅增加了活性位点的暴露,而且赋予其良好的磁回收特性,为开发高效稳定的非均相光催化自fenton系统提供了创新策略,为下一代废水处理提供了可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tailoring Fe 3d spin states for enhanced adsorption/activation of in situ generated H2O2 on S-doped magnetite@resorcinol-formaldehyde resins core-shell catalysts toward boosted cascade self-Fenton oxidation
Although photocatalytic self-Fenton oxidation technology enables sustainable utilization of oxidants through in situ H2O2 generation, the low concentration and sluggish diffusion of such H2O2 on the surface of self-Fenton catalysts substantially restrict the organic pollutant oxidation efficiency in self-Fenton systems. Herein, this study developed a core-shell sulfur-doped magnetite (S-Fe3O4) @ resorcinol-formaldehyde (RF) resins photo-assisted self-Fenton catalyst by tuning the electronic structure of iron-based catalysts through a sulfidation strategy. The introduction of sulfur species promoted the Fe(II)/Fe(III) redox cycle and significantly enhanced the adsorption and activation of H2O2, which is in situ generated from the visible-light-responsible RF resins thin shell. With all the merits above, this catalyst achieved efficient degradation of fluoroquinolone antibiotics, represented by ciprofloxacin (CIP). Radical quenching experiments and electron spin resonance (ESR) analysis identified •OH and •O2− as the dominant reactive oxygen species in the S-Fe3O4@RF-mediated self-Fenton reactions. Systematic characterizations and density functional theory (DFT) calculations revealed that the sulfidation treatment reduced the activation energy barrier of H2O2 to produce •OH by regulating the electronic state density of the iron species in the composite catalysts and the free radical-dominated degradation pathway of the pollutants was also clarified. The unique core-shell structure of the catalyst not only increased the exposure of active sites but also endowed it with a favorable magnetic recovery feature, providing an innovative strategy for the development of efficient and stable heterogeneous photocatalytic self-Fenton systems for the next generation of wastewater treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


