氟烷基取代环丙烯与氟烷基丙烯基硅烷作为羰基阳离子等价物的模块化合成
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
羰基阳离子是一种能够形成三个共价键的反应性物质,由于其极强的反应性和有效来源的有限性,仍然相对未被开发。本文中,我们报道了氟烷基烷基硅烷作为羰基阳离子等价物的应用,使得氟烷基取代环丙烯的模块化合成成为可能,其中构建了三个新的共价键。该反应的成功之处在于将顺序光催化和酸催化在一锅过程中结合起来。光催化促进了碳介导的[2 + 1]与炔烃的环加成反应,而酸催化通过环丙烯中间体促进了正式的C-O键功能化。使用这种策略,我们已经合成了各种氟烷基化环丙烯醚、芳烃、烷烃、硫醚、胺和叠氮化物。这些反应不仅对简单环丙烯的合成有效,而且还提供了制备复杂环丙烯的途径,否则很难制备。本文章由计算机程序翻译,如有差异,请以英文原文为准。
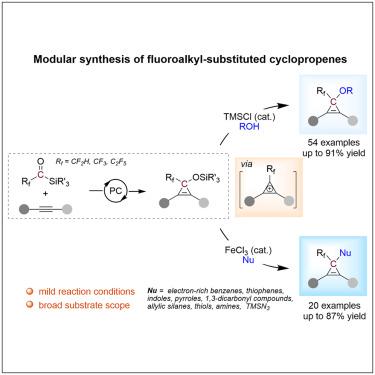
Modular synthesis of fluoroalkyl-substituted cyclopropenes with fluoroalkylacylsilanes as carbynyl cation equivalents
The carbynyl cation, which is a reactive species capable of forming three covalent bonds, remains relatively unexplored due to its extreme reactivity and the limited availability of efficient sources. Herein, we report the application of fluoroalkylacylsilanes as carbynyl cation equivalents, enabling the modular synthesis of fluoroalkyl-substituted cyclopropenes, where three new covalent bonds are constructed. The success of this reaction lies in the integration of sequential photocatalysis and acid catalysis in a one-pot process. Photocatalysis facilitates the carbene-mediated [2 + 1] cycloaddition with alkynes, while acid catalysis promotes formal C–O bond functionalization via cyclopropenium intermediates. Using this strategy, we have synthesized a diverse array of fluoroalkylated cyclopropenyl ethers, arenes, alkanes, thioethers, amines, and azides. These reactions are not only effective for the synthesis of simple cyclopropenes but also provide access to complex cyclopropenes that are otherwise challenging to prepare.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


