自然获得的启动子变异影响肺炎链球菌感染的结果
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
肺炎链球菌定植于人的呼吸道,在那里它通过糖苷水解酶和糖运输系统从糖基化的粘蛋白中获取糖。本研究确定了编码多糖清除系统的肺炎球菌操纵子启动子中广泛存在的核苷酸序列变异。我们在21,155个基因组中鉴定了78个启动子序列模式,变异聚集在一段腺嘌呤中,在DNA复制过程中,突变通过链滑移积累。启动子突变影响操纵子转录,并且在单载体事件中多个启动子模式被共同识别,这表明异质基因表达提供了群体水平的益处。在小鼠鼻咽定植模型中,启动子突变产生并经历选择,核苷酸插入促进基因表达并延长载体寿命。预先存在的免疫可以抵抗携带单一启动子模式的菌株的定植,但不能抵抗与启动子序列不同的其他等基因菌株的混合感染。启动子区序列变异为探索表型空间以最大化宿主内适应度提供了一种进化策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
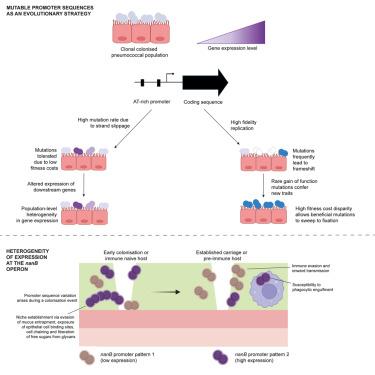
Naturally acquired promoter variation influences Streptococcus pneumoniae infection outcomes
Streptococcus pneumoniae colonizes human airways, where it acquires sugars from glycosylated mucins using glycoside hydrolases and sugar transport systems. This study identifies widespread nucleotide sequence variation in the promoter of a pneumococcal operon encoding a glycan scavenging system. We identify 78 promoter sequence patterns across 21,155 genomes, with variation clustered within a stretch of adenines, where mutations accumulate via strand slippage during DNA replication. Promoter mutations influence operon transcription, and multiple promoter patterns are co-identified during single-carriage episodes, suggesting that heterogeneous gene expression provides population-level benefits. In a mouse nasopharyngeal colonization model, promoter mutations arise and undergo selection, with nucleotide insertion promoting gene expression and prolonging carriage longevity. Pre-existing immunity confers resistance to colonization by strains carrying single promoter patterns but does not protect against mixed infections with otherwise isogenic strains differing in promoter sequence. Promoter region sequence variation offers an evolutionary strategy for exploration of phenotypic space to maximize fitness within-host.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


