从废锂离子电池中精确分离Ni/Co:揭示H2O在深共晶溶剂中的关键作用
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
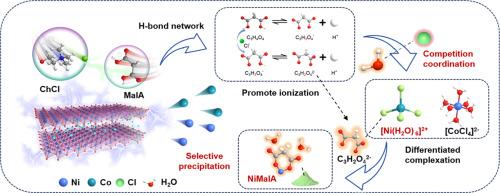
从报废锂离子电池(LIBs)中可持续回收有价金属已成为缓解关键资源供需矛盾的关键。然而,过渡金属在物理化学性质上的高度相似性给选择性回收带来了根本性的挑战。在这里,我们提出了一个简单的策略来确定沉淀/溶解行为的分界线,以实现精确的Ni/Co分离。研制了丙二酸基深度共晶溶剂(DES),强调h2o介导的竞争配位和差异化溶解度。在DES-20% H2O体系中,我们发现了[Ni(H2O)6]2+和[CoCl4]2-的独特形成。此外,在富含C3H2O42的环境中,ni -配合物和co -配合物的Ksp差异被有效放大。这些显著的差异协同产生C3H2NiO4·2H2O沉淀(纯度约99.9%),实现了显著的Ni/Co分离因子125,保证了所有金属的顺序分离。这种通用的方法以及深入的机制理解为lib的增值开采提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Precise Ni/Co separation from spent Li-Ion batteries: Revealing the pivotal role of H2O in deep eutectic solvents
The sustainable recovery of valuable metals from end-of-life lithium-ion batteries (LIBs) has become essential for relieving the supply-demand contradiction of key resources. However, the high similarity in physicochemical properties of transition metals poses a fundamental challenge for the selective recycling. Here we proposed a facile strategy for defining the demarcation line of precipitation/dissolution behavior for precise Ni/Co separation. A malonic acid-based deep eutectic solvent (DES) is developed, emphasizing the H2O-mediated competitive coordination and differentiated solubility. We discovered the distinctive formation of [Ni(H2O)6]2+ and [CoCl4]2- in the DES-20 % H2O system. Furthermore, the Ksp differences of the Ni-complex and Co-complex in the C3H2O42--rich environment was effectively magnified. These significant differences synergistically enable the generation of C3H2NiO4·2H2O precipitation (∼ 99.9 % purity), achieving a remarkable Ni/Co separation factor of 125, which guarantees sequential separation of all metals. This universal methodology along with in-depth mechanism understanding provides a guideline toward value-added recovery of LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


