CuOx/Cu(110)催化水气转换反应的综合理论研究
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
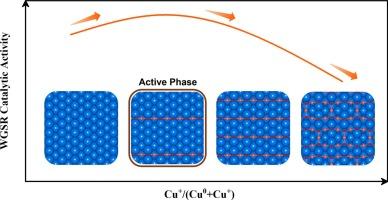
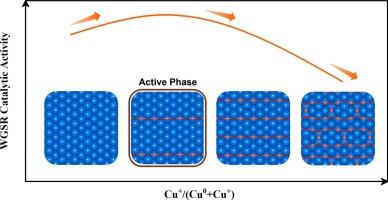
近年来,国内外对铜基水气转换反应催化剂的理论研究得到了广泛的开展。然而,铜原子在反应条件下的氧化态及其在催化WGSR中的作用等问题仍未得到解决。表面科学实验表明,干净的Cu(110)比干净的Cu(111)具有更高的催化活性,并且先前的研究从理论上表明氧化可以提高Cu(111)的内在活性。因此,确定Cu(110)表面氧化物及其催化活性是基础,并将为合理的催化剂设计提供有价值的见解。本文采用从头算原子热力学方法揭示了从清洁Cu(110)到Cu2O(110)的潜在相变,包括添加的row-(4 × 1)(AR-41)、添加的row-(2 × 1)(AR-21)以及添加的链c(6 × 2)(as -c62)结构。利用密度泛函理论(DFT)计算系统地研究了这些不同表面上WGSR的机理。此外,通过平均场微动力学模型(MF-MKM)和动力学蒙特卡罗(kMC)模拟,我们发现催化性能与Cu(110)的氧化程度之间存在火山型关系,轻度氧化的AR-41结构表现出最佳活性。此外,速率控制程度(DRC)分析显示,与清洁Cu相比,水解离不再是氧化结构的速率决定步骤(110)。相反,CO的直接氧化和H2的形成可能在CuOx/Cu(110)催化的WGSR中发挥更关键的作用。同时发现氧化还原机制和羧基机制是相互竞争的。羧基机制主要在低水平氧化时起作用,而氧化还原机制在高水平氧化时起优先作用。因此,适当的氧化态将增强对Cu(110)的催化活性。此外,掺杂其他金属原子作为提高催化剂性能的策略,发现掺杂Pd或Zn可以略微提高催化剂的催化性能。此外,对CuOx/Cu(110)和CuOx/Cu(111)的催化活性进行了比较,发现它们由于CuOx与金属亚表面的独特界面而表现出不同的催化活性本文章由计算机程序翻译,如有差异,请以英文原文为准。
A comprehensive theoretical study on CuOx/Cu(110) catalyzed water–gas shift reaction
In recent years, theoretical studies on Cu-based catalysts for the water–gas shift reaction (WGSR) have been conducted extensively. However, the issues concerning the oxidation state of copper atoms under reaction conditions and their roles in catalyzing WGSR remain unresolved. Surface science experiments have demonstrated that clean Cu(110) exhibits higher catalytic activity than clean Cu(111), and a previous study has theoretically indicated that the oxidation can enhance the intrinsic activity of Cu(111). Therefore, determining the surface oxides on Cu(110) and their catalytic activity is fundamental and will provide valuable insights for rational catalyst design. In this paper, ab initio atomistic thermodynamics was performed to reveal the potential phase transitions from clean Cu(110) to Cu2O (110), including added row-(4 × 1) (AR-41), added row-(2 × 1) (AR-21), as well as added strand-c(6 × 2) (AS-c62) structures. The WGSR mechanisms were systematically investigated on these distinct surfaces by density functional theory (DFT) calculations. Additionally, we identified a volcano-type relationship between the catalytic performance and the oxidation degree of Cu(110), with the mildly oxidized AR-41 structure exhibiting optimal activity, as determined by mean-field microkinetic modeling (MF-MKM) and kinetic Monte Carlo (kMC) simulations. Moreover, the degree of rate control (DRC) analysis revealed that water dissociation is no longer the rate-determining step for oxidized structures compared to clean Cu(110). Instead, the direct oxidation of CO and the production of H2 may play a more critical role in CuOx/Cu(110)-catalyzed WGSR. Meanwhile, it was found that the redox and carboxyl mechanisms compete with each other. The carboxyl mechanism predominantly operates at low levels of oxidation, while the redox mechanism takes precedence at high levels of oxidation. Consequently, the appropriate oxidation state will enhance catalytic activity on Cu(110). Additionally, doping with other metal atoms was used as a strategy to improve catalyst performance, and it was found that doping with Pd or Zn may slightly promote the catalytic performance. Furthermore, a comparison between CuOx/Cu(110) and CuOx/Cu(111) was conducted, and it was found that they show different catalytic activity due to their unique interfaces of CuOx and metallic subsurface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


