p53信号通路中USP28细胞功能的药理学研究
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
去泛素化酶(DUBs)是泛素信号传导和蛋白质降解的关键调节因子,由于缺乏高质量的化学探针,人们对其仍不完全了解。为了解决这一挑战,我们开发了CAS-010,这是一种低纳摩尔的USP28泛素竞争性抑制剂,它对USP28的活性优于其他dub,同时对密切相关的USP25也有一定的活性。我们合理化了我们的SAR趋势,并使用USP28与抑制剂配合的晶体结构观察了选择性。我们验证了CAS-010在野生型环境下对p53转激活的负调控作用。我们证明了CAS-010破坏了53BP1-USP28的相互作用,并且更广泛地表明USP28的催化活性有助于这种关键的相互作用。综上所述,CAS-010和伴随的阴性对照化合物WPT-086以及抑制剂抗性突变体为进一步表征USP28在p53介导的细胞周期控制和细胞命运中的作用提供了良好的验证工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
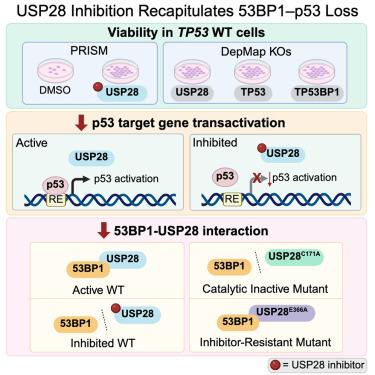
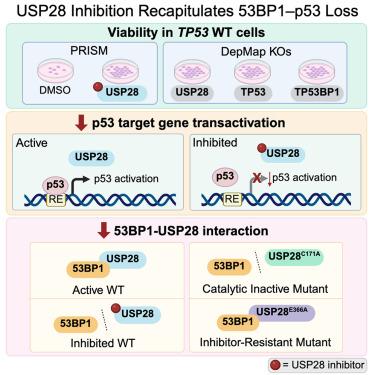
Pharmacologic interrogation of USP28 cellular function in p53 signaling
Deubiquitinating enzymes (DUBs) are crucial regulators of ubiquitin signaling and protein degradation that remain incompletely understood in part due to the lack of high-quality chemical probes. To address this challenge, we developed CAS-010, a low nanomolar, ubiquitin-competitive inhibitor of USP28 that demonstrates preferential activity against USP28 over other DUBs, while also exhibiting some activity against the closely related USP25. We rationalized our SAR trends and observed selectivity using a crystal structure of USP28 in complex with an inhibitor. We validated on-target effects of CAS-010 on the negative regulation of p53 transactivation in the wild-type setting. We demonstrated that CAS-010 disrupts the 53BP1-USP28 interaction, and more broadly showed that USP28 catalytic activity contributes to this key interaction. Taken together, CAS-010 and the accompanying negative control compound WPT-086 and inhibitor-resistant mutant provide well-validated tools for further characterizing the role of USP28 in p53-mediated effect on cell cycle control and cell fate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


