自闭症相关蛋白形成复合体,维持小鼠纹状体的不对称性
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
大脑的两个半球表现出深刻的偏侧化,但其潜在的机制仍然难以捉摸。通过对双侧纹状体的蛋白质组学和磷酸化蛋白质组学分析,我们发现了显著的磷酸化不对称。双侧纹状体是重要脑功能的中心,也是自闭症病理生理的共同节点。特别是,左纹状体的磷酸化过程似乎更容易受到干扰。值得注意的是,SH3RF2,其单拷贝敲除导致小鼠自闭症谱系障碍(ASD)样行为,在纹状体中唯一表达,与CaMKII(一种ASD相关蛋白)和PPP1CC形成复合物。SH3RF2的缺失扰乱了CaMKII/PP1“开关”,导致CaMKII过度活跃,其底物GluR1磷酸化增加。在sh3rp2缺失的小鼠中,左侧纹状体中GluR1-Ser831磷酸化的升高及其突触后膜定位的异常可能会损害纹状体神经元的功能侧化,并导致自闭症样行为。这项研究首次揭示了哺乳动物大脑偏侧化的分子机制,将其损伤与自闭症的发展和治疗策略联系起来。本文章由计算机程序翻译,如有差异,请以英文原文为准。

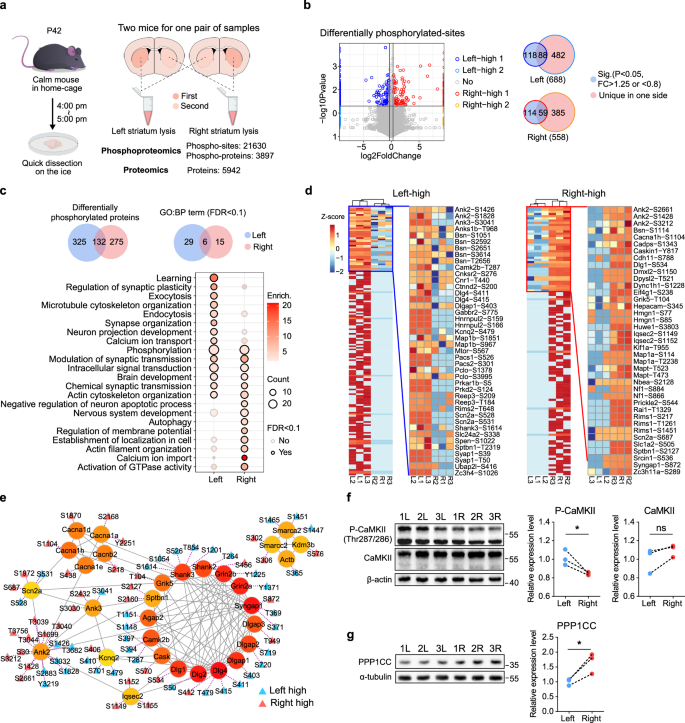
Autism-related proteins form a complex to maintain the striatal asymmetry in mice
The brain’s hemispheres exhibit profound lateralization, yet the underlying mechanisms remain elusive. Using proteomic and phosphoproteomic analyses of the bilateral striatum — a hub for important brain functions and a common node of autism pathophysiology — we identified significant phosphorylation asymmetries. Particularly, the phosphorylation processes in the left striatum appear more prone to disturbance. Notably, SH3RF2, whose single-copy knockout leads to autism spectrum disorder (ASD)-like behaviors in mice, is uniquely expressed in the striatum, forming a complex with CaMKII (an ASD-associated protein) and PPP1CC. Loss of SH3RF2 disturbs the CaMKII/PP1 “switch”, resulting in hyperactivity of CaMKII and increased phosphorylation of its substrate GluR1. In Sh3rf2-deficient mice, heightened GluR1-Ser831 phosphorylation and its aberrant postsynaptic membrane localization in the left striatum may impair the functional lateralization of striatal neurons and contribute to autism-like behaviors. This study unveils the first molecular mechanism governing brain lateralization in mammals, linking its impairment to autism development and treatment strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


