评估全氟烷基物质对肝脏健康的影响:一项使用多供体人肝球体的综合研究
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
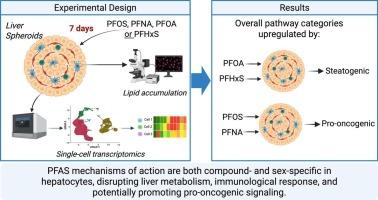
虽然全氟烷基和多氟烷基物质(PFAS)与慢性肝病有关,但不同PFAS导致人类肝功能障碍的具体细胞和分子机制尚不清楚。本研究旨在阐明这些机制。方法将由多种细胞类型组成的多供体人肝脏球体模型暴露于20 µM的PFHxS、PFOA、PFOS或PFNA中7天,然后进行单细胞RNA测序和脂质染色。结果spfas对肝球体的影响具有复合性和性别特异性。PFOA和PFHxS增加了脂质积累,而PFOS和PFNA触发了多种与癌症相关的途径。PFOA上调新生脂肪生成,特别是在女性源性肝细胞中,而PFHxS下调所有肝细胞的脂质运输和外排途径。在两性肝细胞中,PFNA上调了参与细胞周期进程、氧化应激、DNA修复和炎症的途径。值得注意的是,61.3%暴露于pfna的细胞表达了转录组癌特征。全氟辛烷磺酸主要影响男性来源的肝细胞,表现出轻微的影响。所有化合物都破坏T/NK细胞和Kupffer细胞的免疫相关通路。此外,PFAS暴露减少了细胞间的交流,引发了涉及血管生成、细胞凋亡、细胞增殖和粘附、脂质代谢和炎症的细胞相互作用。结论PFAS破坏肝脏代谢,并可能通过化合物和性别特异性机制促进促癌信号传导。这些见解增强了我们对PFAS肝毒性的理解,并强调了在未来的毒理学和公共卫生评估中将性别作为生物学变量的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Assessing the impact of perfluoroalkyl substances on liver health: a comprehensive study using multi-donor human liver spheroids
Background
Although per- and polyfluoroalkyl substances (PFAS) have been linked to chronic liver diseases, the specific cellular and molecular mechanisms by which different PFAS contribute to human liver dysfunction remain unclear. This study aims to elucidate those mechanisms.
Methods
We exposed a multi-donor human liver spheroid model composed of multiple cell types to 20 µM of PFHxS, PFOA, PFOS, or PFNA for seven days, followed by single-cell RNA sequencing and lipid staining.
Results
PFAS impacted liver spheroids in a compound- and sex-specific manner. PFOA and PFHxS increased lipid accumulation, while PFOS and PFNA triggered multiple cancer-related pathways. PFOA upregulated de novo lipogenesis, particularly in female-derived hepatocytes, whereas PFHxS downregulated lipid transportation and efflux pathways across all hepatocytes. PFNA upregulated pathways involved in cell cycle progression, oxidative stress, DNA repair, and inflammation in hepatocytes from both sexes. Notably, 61.3% of the PFNA-exposed cells expressed a transcriptomic cancer signature. PFOS predominantly affected male-derived hepatocytes, showing a mild effect. All compounds impaired immune-related pathways in T/NK and Kupffer cells. Furthermore, PFAS exposure reduced cell–cell communication and elicited cellular interactions involved in angiogenesis, apoptosis, cell proliferation and adhesion, lipid metabolism, and inflammation.
Conclusions
Our findings suggest that PFAS disrupt liver metabolism and may promote pro-oncogenic signaling through compound- and sex-specific mechanisms. These insights enhance our understanding of PFAS hepatotoxicity and underscore the importance of considering sex as a biological variable in future toxicological and public health assessments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


