Al掺杂与溶胶-凝胶合成对环丙沙星光催化降解的协同作用
摘要
本研究首次研究了Al掺杂Mn0.3Ba0.4Cd0.3AlxFe2-xO4对环丙沙星的光催化降解,考察了Al掺杂对钡锰铁氧体光催化性能的影响,以及Mn0.3Ba0.4Cd0.3AlxFe2-xO4 (x = 0.0, 0.5)尖晶石铁氧体的结构、光学、表面积和形态性能。以及它们随后对环丙沙星降解的光催化性能。掺杂al的铁氧体达到了93.45% degradation efficiency, outperforming the undoped sample by 56.68%. This enhanced photocatalytic activity can be attributed to the synergistic effects of Al doping, which include a narrowed bandgap, increased surface area, reduced particle size, and modified electronic structure. Scavenger analysis revealed that hydroxyl radicals were the primary reactive species. Al doping decreased the bandgap from 2.2 eV to 1.9 eV and reduced crystallite size. FTIR spectroscopy indicated structural changes, with peak shifts in the tetrahedral and octahedral sites. SEM revealed a refined microstructure with smaller, uniform particles, reducing the average grain size from 61.72 nm to 50.33 nm. The BET surface area increased upon Al doping. Kinetic studies have shown that degradation follows pseudo-first-order kinetics. Photocatalyst Performance Assessment revealed improved quantum yield (\({5\times 10}^{-9}\) molecules/photon) and space-time yield (\({2.50\times 10}^{-10}\) molecules/photon/mg) for the Al-doped sample. The enhanced photocatalytic activity is attributed to the increased surface area, reduced particle size, and modified electronic structure owing to the Al doping. This study highlights the potential of Al-doped Mn0.3Ba0.4Cd0.3AlxFe2-xO4 (x = 0.0, 0.5) ferrites as efficient photocatalysts for ciprofloxacin degradation during water treatment.Graphical Abstract
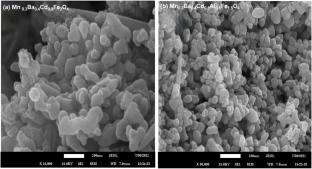
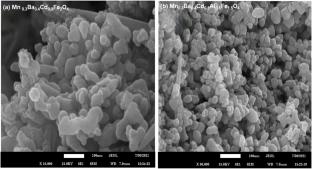
This study is the first to investigate the photocatalytic degradation of ciprofloxacin using Al-doped Mn0.3Ba0.4Cd0.3AlxFe2-xO4 and examine the impact of Al doping on the photocatalytic properties of barium-manganese ferrites, as well as the structural, optical, surface area, and morphological properties of Mn0.3Ba0.4Cd0.3AlxFe2-xO4 (x = 0.0, 0.5) spinel ferrites. and their subsequent photocatalytic performance for ciprofloxacin degradation. The Al-doped ferrite achieved a 93.45% degradation efficiency, outperforming the undoped sample by 56.68%. This enhanced photocatalytic activity can be attributed to the synergistic effects of Al doping, which include a narrowed bandgap, increased surface area, reduced particle size, and modified electronic structure. Scavenger analysis revealed that hydroxyl radicals were the primary reactive species. Al doping decreased the bandgap from 2.2 eV to 1.9 eV and reduced crystallite size. FTIR spectroscopy indicated structural changes, with peak shifts in the tetrahedral and octahedral sites. SEM revealed a refined microstructure with smaller, uniform particles, reducing the average grain size from 61.72 nm to 50.33 nm. The BET surface area increased upon Al doping. Kinetic studies have shown that degradation follows pseudo-first-order kinetics. Photocatalyst Performance Assessment revealed improved quantum yield (\({5\times 10}^{-9}\) molecules/photon) and space-time yield (\({2.50\times 10}^{-10}\) molecules/photon/mg) for the Al-doped sample. The enhanced photocatalytic activity is attributed to the increased surface area, reduced particle size, and modified electronic structure owing to the Al doping. This study highlights the potential of Al-doped Mn0.3Ba0.4Cd0.3AlxFe2-xO4 (x = 0.0, 0.5) ferrites as efficient photocatalysts for ciprofloxacin degradation during water treatment.
Graphical Abstract

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


