不同溶剂极性环境对(E)- n ' -(5-溴-2-羟基苄基)-4-羟基苯并肼分子ESIPT过程的调控
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
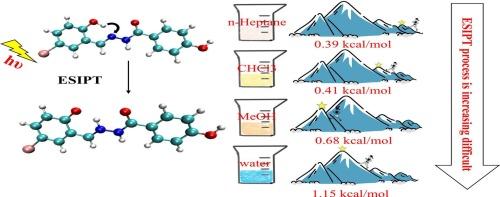
最近,实验合成了一种新型探针,并发现其在不同溶剂中表现出不同的光物理行为。我们的工作重点是通过密度泛函理论(DFT)和时变密度泛函理论(TD-DFT)方法来阐明这一现象的机制。光激发后,四种溶剂中氢键强度均增加,从正庚烷、三氯甲烷、甲醇到水,氢键强度逐渐降低。这一趋势与扫描势能面(PES)得到的能垒变化一致,表明溶剂的极性会影响氢键的强度,从而进一步影响质子转移的难易程度。此外,电子能谱的仿真也验证了理论方法的可靠性。电子-空穴分析证实,在微观水平上,较高的溶剂极性对ESIPT过程有负面影响。本研究为利用激发态分子内质子转移(ESIPT)操作设计和合成荧光探针提供了一些思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Regulation of ESIPT process in (E)-N′-(5-bromo-2-hydroxybenzylidene)-4-hydroxybenzohydrazide molecules by different solvent polarity environments
Recently, a novel probe was synthesized experimentally and found to exhibit different photophysical behaviors in various solvents. Our work focus on elucidating the mechanism underlying this phenomena by density functional theory (DFT) and the time-dependent density functional theory (TD-DFT) methods. The strength of hydrogen bonds increased in all four solvents after photoexcitation, with a gradual decrease from n-Heptane, Trichloromethane (CHCl3), Methanol (MeOH) to water. This trend aligns with the energy barrier changes obtained by scanning the potential energy surface(PES), which indicates that the polarity of the solvent can affect the strength of hydrogen bonds, thereby further influencing the ease of proton transfer. Additionally, the simulation of electron spectra validates the reliability of the theoretical method. The electron-hole analysis confirms that higher solvent polarity negatively affects the ESIPT process at the microscopic level. Our work provides some ideas for designing and synthesizing fluorescent probes through excited state intramolecular proton transfer (ESIPT) operation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


