基于氟化二苯基镧异构体的高迁移率发射n型有机半导体的多尺度研究
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
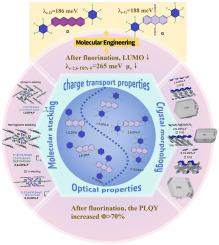
Ntype高迁移率发射型有机半导体材料(HMEOSCs)是推进逻辑电路和传感器的关键。然而,由于固有分子结构和固态堆叠的限制,在实现高载流子迁移率和强发光性能方面,它们的发展面临着巨大的挑战。本研究从理论上设计了一系列氟化二苯基蒽异构体(1,5-、2,6-和9,10- dpa - f),并通过第一性原理计算系统地研究了它们的电荷输运和荧光性质。此外,晶体生长形态建模以建立多尺度结构-性能关系。结果表明,芳基取代基的全氟化可以有效降低LUMO能级,增强电子注入能力,并将荧光量子产率提高到70%以上。然而,它也带来了重组能较大和分子间沿长轴滑动的问题。在此基础上,开发了以2,6- dpa - f为中心的分子再设计策略来抑制电子重组能。此外,我们还通过晶体形态生长阐明了高迁移率分子1,5- dpa - f和9,10- dpa - f (μes>1 cm2V−1s−1)尚未获得实验测量值的原因。这项工作阐明了多尺度因素(分子结构-堆叠-形态)对输运性质的影响,为n型hmeosc的设计开辟了新的途径和视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multi-scale investigation on high mobility emissive n-type organic semiconductors based on fluorinated diphenylanthracene isomers
N
type high mobility emissive organic semiconductor materials (HMEOSCs) are pivotal for advancing logic circuits and sensors. Nevertheless, their development faces the great challenge in achieving both high carrier mobility and strong luminescent properties, attributed to the limitations of intrinsic molecular structure and solid-state stacking. In this study, a series of fluorinated diphenylanthracene isomers (1,5-, 2,6-, and 9,10-DPA-F) were theoretically designed, with their charge transport and fluorescence properties systematically investigated via first-principles calculations. Additionally, crystal growth morphologies were modeled to establish multiscale structure-property relationships. The results show that the perfluorination of aryl substituents were demonstrated to effectively reduce LUMO energy levels, enhance electron injection capabilities, and elevate fluorescence quantum yields beyond 70 %. However, it also brings the problems of larger reorganization energy and intermolecular sliding along the long axis. Building on this foundation, molecular redesign strategies focused on 2,6-DPA-F were developed to suppress electronic reorganization energy. Moreover, we also clarified the reason by crystal morphology growth why high mobility molecules 1,5-DPA-F and 9,10-DPA-F (μes>1 cm2V−1s−1) have not yet obtained experimental measurement values. This work elucidates the influence of multi-scale factors (molecular structure-stacking-morphology) on the transport properties, and opens up new avenues and perspectives for the design of n-type HMEOSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


