叉头盒P1缺失时选择素E的上调增加了高血压脑出血中性粒细胞浸润和脑损伤
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
脑内高血压可导致高血压性脑出血,这是一种毁灭性的疾病。本研究基于生物信息学的见解,旨在探讨选择素E (SELE)和叉头盒P1 (FOXP1)在HICH进展中的功能。通过血管紧张素II和L-NAME处理产生的小鼠脑出血模型的纹状体中检测到SELE表达增加。抑制SELE可减轻小鼠脑出血、中性粒细胞浸润和血脑屏障(BBB)破裂。FOXP1在high小鼠中低表达,通过结合其启动子区域抑制SELE转录。FOXP1的过表达在high小鼠中也有类似的缓解作用;然而,这种效果被额外的SELE过表达所消除。在体外,用凝血酶处理人脑微血管内皮细胞(HBMECs),生成ICH细胞模型,然后与HL-60细胞源性中性粒细胞共培养。FOXP1过表达降低中性粒细胞粘附,减轻HBMEC细胞凋亡和通透性,促进血管生成。然而,这些影响被SELE上调所抵消。总之,本研究表明,SELE对FOXP1缺失的上调与HICH中中性粒细胞浸润和血脑屏障破裂有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
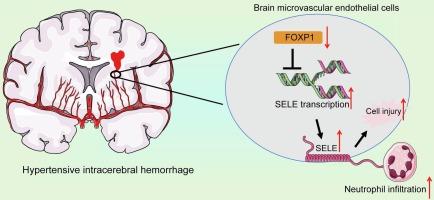
Selectin E upregulation upon forkhead box P1 loss augments neutrophil infiltration and brain damage in hypertensive intracerebral hemorrhage
Hypertension in the brain may lead to hypertensive intracerebral hemorrhage (HICH), a devastating disease. This study, grounded on bioinformatics insights, aims to investigate the functions of Selectin E (SELE) and forkhead box P1 (FOXP1) in the progression of HICH. Increased SELE expression was detected in the striatum of a mouse model of HICH generated via angiotensin II and L-NAME treatments. Knockdown of SELE alleviated hemorrhage, neutrophil infiltration, and blood–brain barrier (BBB) rupture in the mouse brain. FOXP1, poorly expressed in the HICH mice, was found to repress SELE transcription by binding to its promoter region. Overexpression of FOXP1 resulted in analogous alleviating effects in the HICH mice; however, the effects were abrogated by the additional SELE overexpression. In vitro, human brain microvascular endothelial cells (HBMECs) were treated with thrombin to generate a cellular model of ICH, followed by co-culture with HL-60 cell-derived neutrophils. The FOXP1 overexpression reduced the adhesion of neutrophils, and it alleviated HBMEC apoptosis and permeability while enhancing angiogenesis. Still, these effects were counteracted by the SELE upregulation. In conclusion, this study demonstrates that SELE upregulation upon FOXP1 loss is associated with neutrophil infiltration and BBB rupture in HICH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


