助溶剂对抗真菌药硝酸丙环唑与β-环糊精络合的影响:分子动力学和核磁共振相结合的研究
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
了解共溶剂在环糊精(CyD)-药物络合中的作用对于优化药物配方至关重要,因为共溶剂影响溶解度、稳定性和结合亲和力。采用分子动力学(MD)模拟和核磁共振(NMR)技术研究了硝酸丙康唑(PCZHNO₃)与β-环糊精(β-CyD)在混合二甲亚砜(DMSO)/水溶剂体系中的包合作用。MD模拟表明,DMSO对β-CyD空腔具有很强的亲和力,即使在低浓度下也能有效地取代水分子,这突出了DMSO和PCZHNO₃在β-CyD空腔内的竞争性质。增强的采样模拟技术和平均力势(PMF)计算表明,在低DMSO浓度(~ 5% v/v)下,络合作用最有利,随着DMSO水平的增加,结合亲和力逐渐降低,最终导致高DMSO比下复合物的不稳定。1H-NMR和ROESY谱证实了这些发现,化学位移变化和ROESY交叉峰表明随着DMSO浓度的增加,主客体相互作用减弱。这些结果为共溶剂在调节药物- cyd相互作用中的作用提供了分子水平的见解,强调了定制溶剂环境以提高β-CyD包合物在药物应用中的稳定性和有效性的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
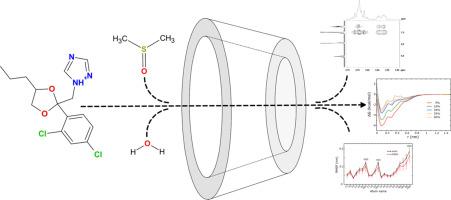
Cosolvent effects on the complexation of the antifungal propiconazole nitrate with β-cyclodextrin: A combined molecular dynamics and NMR study
Understanding the effects of cosolvents in cyclodextrin (CyD)-drug complexation is essential for optimizing drug formulations, as cosolvents influence solubility, stability, and binding affinity. In this study, the inclusion complexation of propiconazole nitrate (PCZH![]() NO₃) with β-cyclodextrin (β-CyD) in mixed dimethyl sulfoxide (DMSO)/water solvent systems was investigated using molecular dynamics (MD) simulations and nuclear magnetic resonance (NMR) spectroscopy. MD simulations revealed that DMSO exhibits a strong affinity for the β-CyD cavity, effectively displacing water molecules even at low concentrations highlighting the competitive nature of DMSO and PCZH
NO₃) with β-cyclodextrin (β-CyD) in mixed dimethyl sulfoxide (DMSO)/water solvent systems was investigated using molecular dynamics (MD) simulations and nuclear magnetic resonance (NMR) spectroscopy. MD simulations revealed that DMSO exhibits a strong affinity for the β-CyD cavity, effectively displacing water molecules even at low concentrations highlighting the competitive nature of DMSO and PCZH![]() NO₃ for inclusion within the β-CyD cavity. Enhanced sampling simulation techniques and potential of mean force (PMF) calculations demonstrated that complexation is most favorable at low DMSO concentrations (∼5 % v/v), with a gradual decrease in binding affinity as DMSO levels increase, ultimately leading to complex destabilization at high DMSO ratios. 1H-NMR and ROESY spectra confirmed these findings, with chemical shift variations and ROESY cross-peaks indicating a weakening of host-guest interactions with increasing DMSO concentration. The results provide molecular-level insights into the role of cosolvents in modulating drug-CyD interactions, highlighting the need to tailor solvent environments to enhance the stability and efficacy of β-CyD inclusion complexes for pharmaceutical applications.
NO₃ for inclusion within the β-CyD cavity. Enhanced sampling simulation techniques and potential of mean force (PMF) calculations demonstrated that complexation is most favorable at low DMSO concentrations (∼5 % v/v), with a gradual decrease in binding affinity as DMSO levels increase, ultimately leading to complex destabilization at high DMSO ratios. 1H-NMR and ROESY spectra confirmed these findings, with chemical shift variations and ROESY cross-peaks indicating a weakening of host-guest interactions with increasing DMSO concentration. The results provide molecular-level insights into the role of cosolvents in modulating drug-CyD interactions, highlighting the need to tailor solvent environments to enhance the stability and efficacy of β-CyD inclusion complexes for pharmaceutical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


