非活化烯烃的区域选择性多组分磺化/环化通过SO2插入获得磺化的六元和七元环胺
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
涉及二氧化硫插入的烯烃自由基级联环化已被证明是获得磺化杂环化合物的一种很有前途的工具,而未活化烯烃的环化则很少被探索。在这里,我们开发了一个三组分的非活性烯烃与二氧化硫和芳基重氮四氟硼酸盐级联反应,通过裂解烯基C-H键生成磺酰基化的四氢吡啶和氮平类。此外,该方案表现出优异的化学和区域选择性以及与广泛的官能团的相容性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

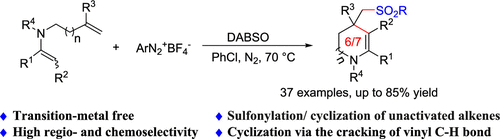
Regioselective Multicomponent Sulfonylation/Cyclization of Unactivated Alkenes to Access Sulfonylated Six- and Seven-Membered Cyclic Enamines via SO2 Insertion
Radical cascade cyclization of alkenes involving the insertion of sulfur dioxide has proven to be a promising tool to access sulfonylnated heterocycle compounds, whereas cyclization of unactivated alkenes has been much less explored. Here, we developed a three-component cascade of unactive alkenes with sulfur dioxide and aryldiazonium tetrafluoroborates to generate sulfonylated tetrahydropyridines and azepines via the cleavage of alkenyl C–H bonds. Moreover, this protocol exhibited excellent chemical and regioselectivity and compatibility with broad functional groups.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


