小介孔杂原子掺杂碳材料的电容性分析
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
小介孔(2 ~ 4 nm)杂原子掺杂碳材料的电荷存储通过双层电容和伪电容两种方式实现。然而,由于对电解质离子如何影响其电容性行为的认识不明确,很难形成统一的设计原则来指导电解质的选择,以促进超级电容器的发展。本文以含氧基团丰富、平均孔径为2.5 nm的一体化碳电极为模型电极,研究了这类碳材料的电容性行为。研究发现,在醋酸铵水溶液中,模型电极的比电容显著增加,电荷存储以离子交换机制为主。由于抑制了碳表面第一吸附层的“过筛选”效应,离子半径较大的乙酸阴离子在尺寸上能更好地匹配较小的介孔,从而增加双层电容。此外,铵态阳离子与含氧基团形成氢键,最大化了与法相反应相关的吉布斯自由能变化,增强了赝电容。本研究阐述了电解质离子对小介孔杂原子掺杂碳材料电容的影响,为高性能水性超级电容器的设计提供了新的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
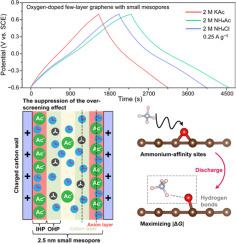
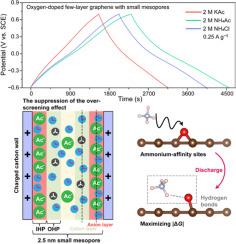
Deciphering the capacitive behavior of heteroatom–doped carbon materials with small mesopores
The charge storage processes of heteroatom–doped carbon materials with small mesopores (2 ∼ 4 nm) occur by both double–layer capacitance and pseudocapacitance. Yet, owing to the ambiguous understanding on how electrolyte ions affect their capacitive behavior, it is hard to form a unified design principle to guide the selection of electrolytes, with the purpose of boosting supercapacitors. Herein, an all–in–one carbon electrode with abundant oxygen–containing groups and an average pore size of 2.5 nm was used as a model electrode to investigate the capacitive behavior of this class of carbon materials. It is found that in ammonium acetate aqueous electrolyte the model electrode shows a significantly increased specific capacitance, and an ion exchange mechanism dominates charge storage. Acetate anions with a larger ion radius can better match small mesopores in size and thus increase double–layer capacitance due to the suppression of the “over–screening” effect in the first adsorbed layer on carbon surfaces. Besides, ammonium cations forms hydrogen bonds with oxygen–containing groups maximizing the Gibbs free energy change related to faradic reactions, which enhances pseudocapacitance. Our work elaborated the effect of electrolyte ions on the capacitance of heteroatom–doped carbon materials with small mesopores and provided new understanding on designing high–performance aqueous supercapacitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


