β-1,3-葡聚糖合成酶样5的失活赋予十字花科植物对芸苔病的广谱抗性
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
棍棒病是由细胞内专性根瘤菌brassicae引起的一种严重危害十字花科作物的病害。广泛分布的田间油菜病型经常克服现代品种的病型特异性抗性,对持久控制该疾病构成挑战。本研究对244份基因组测序的甘蓝型油菜进行了为期3年的全基因组关联研究,发现β-1,3-葡聚糖合成酶样5 (GSL5)与甘蓝型油菜的易感性密切相关。GSL5具有进化保守性,通过基因组编辑在拟南芥、甘蓝型油菜、油菜和甘蓝中灭活GSL5,使甘蓝型油菜在不影响产量的情况下对甘蓝型油菜具有广谱、高水平的抗性。GSL5失活可抑制甘蓝继发性感染过程中茉莉酸介导的免疫,这种免疫抑制可能是通过甘蓝效应物对GSL5的稳定而增强的,从而促进了甘蓝的敏感性。本研究为十字花科根茎病害防治提供了持久的抗性资源,并对植物对细胞内真核植物病原体的抗性有了新的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。

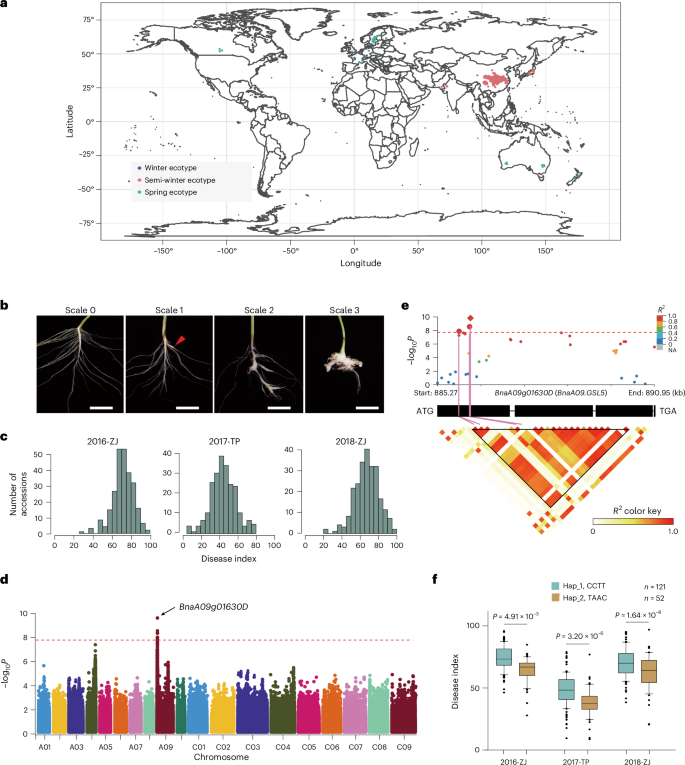
Inactivation of β-1,3-glucan synthase-like 5 confers broad-spectrum resistance to Plasmodiophora brassicae pathotypes in cruciferous plants
Clubroot disease, caused by the obligate intracellular rhizarian protist Plasmodiophora brassicae, is devastating to cruciferous crops worldwide. Widespread field P. brassicae pathotypes frequently overcome the pathotype-specific resistance of modern varieties, posing a challenge for durable control of this disease. Here a genome-wide association study of 3 years of data comprising field clubroot phenotyping of 244 genome-resequenced Brassica napus accessions identified a strong association of β-1,3-glucan synthase-like 5 (GSL5) with clubroot susceptibility. GSL5 was evolutionarily conserved, and inactivation of GSL5 by genome editing in Arabidopsis, B. napus, Brassica rapa and Brassica oleracea conferred broad-spectrum, high-level resistance to P. brassicae pathotypes without yield penalties in B. napus. GSL5 inactivation derepressed the jasmonic acid-mediated immunity during P. brassicae secondary infection, and this immune repression was possibly reinforced through stabilization of GSL5 by a P. brassicae effector, facilitating clubroot susceptibility. Our study provides durable resistance resources for cruciferous clubroot disease control and insights into plant resistance against intracellular eukaryotic phytopathogens. This study implicates GSL5 inactivation in high, broad-spectrum resistance to the clubroot pathogen Plasmodiophora brassicae in Arabidopsis thaliana, Brassica napus, Brassica oleracea and Brassica rapa.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


