研究β功能化中性和阳离子中四苯基卟啉的激发态动力学和双光子吸收
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-08-25
DOI:10.1016/j.jphotochem.2025.116724
引用次数: 0
摘要
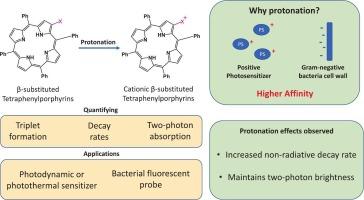
对近年来发展的中四苯基卟啉-吡咯烷/吡咯啉偶联物进行了光动力学失活的光物理表征,包括中性和阳离子衍生物。阳离子化合物在吸收和发射光谱中都表现出红移,同时吸收强度降低,可能反映了构象畸变。荧光量子产率从0.6%到7.1%不等,阳离子卟啉的发射率始终低于中性类似物,其荧光寿命也同样减少。使用连续激光脉冲激发(CLPE)方法测定了三重态量子产率,中性衍生物达到34%,阳离子衍生物达到26%。内部转换是所有导数的主要衰变途径。根据计算的速率,阳离子衍生物的荧光和三重态形成的减少主要是由于非辐射衰变路径的增加。碘离子引起的重原子效应可能是造成这种效应的主要原因之一。使用基于荧光的技术确定了所有样品的双光子吸收横截面光谱。在820 nm和1400 nm之间的测量表明,双光子吸收可以实现与单光子吸收相同的跃迁。这种行为表明分子中存在由溶剂和外围取代基引起的对称性破坏效应。在800 nm附近观察到一个增强的双光子吸收区域,与第一个生物窗口对齐,使其在生物医学应用中特别有前景。虽然电荷的加入降低了样品的单光子吸收值,但对样品的双光子吸收截面和双光子亮度的影响很小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigating the excited state dynamics and two-photon absorption of β-functionalized neutral and cationic meso-tetraphenylporphyrins
Photophysical characterization of recently developed meso-tetraphenylporphyrin-pyrrolidine/pyrroline conjugates with potential in photodynamic inactivation was performed, including both neutral and cationic derivatives. Cationic compounds exhibited red-shifts in both absorption and emission spectra, alongside reduced absorption intensities, likely reflecting conformational distortions. Fluorescence quantum yields ranged from 0.6 % to 7.1 %, with cationic porphyrins consistently less emissive than their neutral analogues, and their fluorescence lifetimes were likewise decreased. Triplet quantum yields were determined using the Consecutive Laser Pulse Excitation (CLPE) method, reaching up to 34 % for neutral and 26 % for cationic derivatives. Internal conversion was the dominant decay pathway across all the derivatives. According to the calculated rates, the decrease in fluorescence and triplet formation observed for cationic derivatives was mostly due to the increase in the non-radiative decay path. Heavy-atom effect promoted by the iodide counterion may be one of the main causes for this effect. Two-photon absorption cross-section spectra were determined for all the samples using a fluorescence-based technique. Measurements between 820 nm and 1400 nm revealed that two-photon absorption enables the same transitions as one-photon absorption. This behavior indicates a symmetry-breaking effect in the molecule, induced by the solvent and peripheral substituents. A region of enhanced two-photon absorption was observed near 800 nm, aligning with the first biological window, making it particularly promising for biomedical applications. While the added charge reduced one-photon absorption values, it had minimal impact on the two-photon absorption cross-section or two-photon brightness of these samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


