MALAT1通过miR-422a/NUP155轴提高变应性鼻炎小鼠Th2细胞因子的分泌
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
MALAT1是在多种疾病中研究最多的lncrna之一。本研究试图探讨长链非编码RNA MALAT1是否参与变应性鼻炎(AR)的发生发展。在这项工作中,从AR患者获得鼻黏膜组织。采用卵清蛋白致敏和攻毒法建立小鼠AR模型。在AR患者和小鼠中,MALAT1、GATA3和NUP155上调,miR-422a下调。与正常小鼠相比,AR小鼠的打喷嚏次数、摩擦次数和流鼻涕次数均有所增加。AR小鼠鼻灌洗液中IL-4、IL-5、IL-9、IL-13水平升高。MALAT1缺乏抑制ILC2细胞中Th2细胞因子的分泌,而NUP155过表达则消除了这一作用。NUP155敲低逆转了MALAT1过表达对Th2细胞因子分泌的促进作用。此外,MALAT1通过海绵miR-422a提高NUP155的表达。在体内,MALAT1沉默减少了AR小鼠的打喷嚏次数、摩擦次数和鼻漏,改善了鼻黏膜组织的损伤。同样,NUP155基因敲低也能显著缓解AR临床症状,改善鼻黏膜病理,抑制体内Th2细胞因子的分泌。综上所述,本研究表明MALAT1通过调节miR-422a/NUP155轴提高ILC2细胞中Th2细胞因子的分泌,从而促进AR的进展。因此,MALAT1可能是AR治疗的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
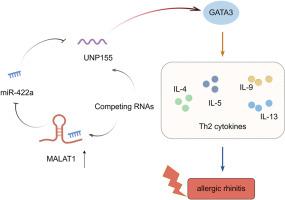
MALAT1 elevates the secretion of Th2 cytokines via miR-422a/NUP155 axis in allergic rhinitis mice
MALAT1 is one of the most well-studied lncRNAs in various diseases. This work attempted to investigate whether long non-coding RNA MALAT1 participate in the development of allergic rhinitis (AR). In this work, nasal mucosal tissues were obtained from AR patients. The mouse AR model was established by sensitization and challenge with ovalbumin. MALAT1, GATA3 and NUP155 were up-regulated, miR-422a was down-regulated in AR patients and mice. The sneezing number, rubbing number and rhinorrhea were increased in AR mice as compared with normal mice. The levels of IL-4, IL-5, IL-9 and IL-13 were elevated in nasal lavage fluid of AR mice. MALAT1 deficiency repressed the secretion of Th2 cytokines in ILC2 cells, which was abrogated by NUP155 overexpression. NUP155 knockdown reversed the promotion of MALAT1 overexpression on the secretion of Th2 cytokines. Moreover, MALAT1 elevated NUP155 expression by sponging miR-422a. In vivo, MALAT1 silencing reduced the sneezing number, rubbing number and rhinorrhea, ameliorated damage of nasal mucosal tissues in AR mice. Similarly, NUP155 knockdown also significantly alleviated AR clinical symptoms, improved nasal mucosal pathology, and suppressed the secretion of Th2 cytokines in vivo. In conclusion, this work demonstrated that MALAT1 elevated the secretion of Th2 cytokines in ILC2 cells by regulating miR-422a/NUP155 axis, which contributed to AR progression. Thus, MALAT1 may be a potential target for AR treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


