产前暴露于2,2 ',4,4 ' -四溴联苯醚对大鼠后代精子功能和DNA甲基化的表观遗传跨代影响
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
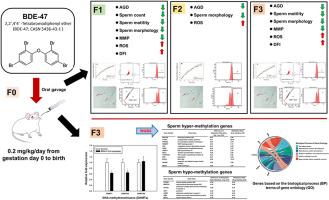
近几十年来,包括2,2 ',4,4 ' -四溴联苯醚(BDE-47)在内的多溴联苯醚的全球生产和使用已大幅减少。然而,BDE-47在世界各地的环境基质和人体组织中仍然普遍存在。在这项研究中,我们研究了产前暴露于BDE-47是否会破坏大鼠后代的精子功能和DNA甲基化。妊娠大鼠从妊娠第0天至分娩期间给予BDE-47治疗。对精子数量、活力、形态、线粒体膜电位(MMP)、活性氧(ROS)产生、精子染色质DNA片段化指数(DFI)、血清睾酮和组织病理学进行代际评估。通过睾丸DNA甲基转移酶表达和全基因组亚硫酸盐测序来确定F3代的DNA甲基化。BDE-47暴露改变了F1代的肛门生殖器距离(AGD)、精子数量、活力、形态、MMP、ROS生成、平均DFI和%DFI;F2代的AGD、形态和ROS的产生;F3代的AGD、运动性、形态学、MMP、ROS生成、平均DFI、%DFI和睾丸DNA甲基转移酶表达。基因本体分析显示SYCP2、ASMT和MSH4与性别分化和生殖发育相关。我们的研究结果表明,产前暴露于BDE-47具有跨代表观遗传效应,诱导男性生殖系统的表型变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Epigenetic transgenerational effects of prenatal exposure to 2,2′,4,4′-tetrabromodiphenyl ether on sperm function and DNA methylation in rat offspring
The global production and use of polybrominated diphenyl ethers, including 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), have been substantially curtailed in recent decades. However, BDE-47 remains ubiquitously detectable in environmental matrices and human tissues worldwide. In this study, we investigated whether prenatal exposure to BDE-47 disrupts sperm function and DNA methylation in rat offspring. Pregnant rats were treated with BDE-47 from gestational day 0 to parturition. Sperm count, motility, morphology, mitochondrial membrane potential (MMP), reactive oxygen species (ROS) production, sperm chromatin DNA fragmentation index (DFI), serum testosterone, and histopathology were evaluated across generations. Testicular DNA methyltransferase expression and whole-genome bisulfite sequencing were performed to determine the DNA methylation in the F3 generation. BDE-47 exposure altered anogenital distance (AGD), sperm count, motility, morphology, MMP, ROS production, mean DFI, and %DFI in the F1 generation; AGD, morphology, and ROS production in the F2 generation; and AGD, motility, morphology, MMP, ROS production, mean DFI, %DFI, and testicular DNA methyltransferase expression in the F3 generation. Gene ontology analysis revealed that SYCP2, ASMT, and MSH4 were associated with sex differentiation and reproductive development. Our findings indicate that prenatal exposure to BDE-47 exerts transgenerational epigenetic effects, inducing phenotypic changes in the male reproductive system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


