百里香-三唑缀合物抗增殖的合成、表征及生物学评价
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文研究了以胸腺苷酸合酶为靶点的新型百里香-三唑杂合体的制备及其抗增殖活性。利用核磁共振、红外和质谱技术对新制备的杂化物的结构进行了表征。结果表明,百里香-三唑复合物对HepG2腺癌的杀伤作用最强。其中化合物12对MCF-7、HepG2和HCT-116的IC50分别为3.52、1.02和4.12 μM,对TS的抑制IC50分别为0.21 μM。此外,化合物12通过将细胞数量从1.14增加到84.07,抑制G1和S期,使细胞周期阻滞在G2期,96%的细胞晚期凋亡。生物学结果也与对接研究相关,在对接研究中,化合物12表现出与5-氟尿嘧啶相似的结合相互作用。这些结果证实了化合物12在癌症治疗中是一种有效的TS抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
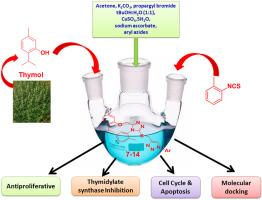
Synthesis, characterization and biological evaluation of thymol-triazole conjugates as antiproliferative agents
The present work focuses on the preparation and antiproliferative activity of new thymol-triazole hybrids targeting thymidylate synthase. The structure of newly prepared hybrids was elucidated using NMR, IR and Mass spectroscopic techniques. The anticancer results showed that the thymol-triazole hybrids were most cytotoxic against HepG2 adenocarcinomas. Among the synthesized hybrids, compound 12 exhibited significant cytotoxic effect towards MCF-7, HepG2 and HCT-116 with IC50 of 3.52, 1.02 and 4.12 μM, respectively as well as TS inhibition with IC50 0.21 μM. Furthermore, compound 12 arrested cell cycle at G2 phase by increasing cell population from 1.14 to 84.07 and suppressing G1 and S stages, with 96 % late apoptosis The biological results were also correlated with docking studies in which compound 12 displayed similar binding interaction to 5-florouracil. These results established compound 12 to be a potent TS inhibitor in cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


