空心氢氧化铁纳米管作为电催化剂用于改进中性析氧反应
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
研究高效、经济的电化学水氧化催化剂已经引起了人们的广泛关注。为此,采用水热法合成了电催化剂氢氧化铁纳米管(Fe-Co - ho - nt),并对其在中性介质中作为析氧反应(OER)催化剂的潜力进行了系统评价。其中,经过6 h合成的Fe-Co-HO-NT-6在水氧化过程中表现出优异的电催化性能。这种增强的性能主要归因于材料的几个有利特性,包括其相对较高的比表面积,优异的导电性和丰富的活性位点的存在。实验结果表明,Fe-Co-HO-NT-6 h的析氧电催化剂表现出优异的催化性能,在极低的过电位(仅为476 mV)下实现了10 mA/cm2的电流密度。该研究为开发高性能电化学水氧化催化剂提供了新的参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
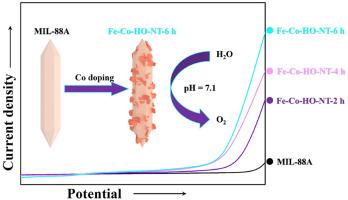
Hollow Fe–Co hydroxide nanotube as an electrocatalyst for an improved neutral oxygen evolution reaction
Investigations into efficient and cost-effective catalysts for the electrochemical water oxidation have garnered significant attention. Therefore, the electrocatalyst Fe–Co hydroxide nanotube (Fe–Co–HO-NT) was synthesized via a straightforward hydrothermal method and its potential as an oxygen evolution reaction (OER) catalyst in a neutral medium was systematically evaluated. Among the samples, the Fe–Co–HO-NT-6 product synthesized over a 6-h period exhibits superior electrocatalytic performance in water oxidation. This enhanced performance can be primarily attributed to several favorable characteristics of the material, including its relatively high specific surface area, excellent electrical conductivity, and the presence of abundant active sites. The experimental results demonstrate that the developed oxygen-evolving electrocatalyst, Fe–Co–HO-NT-6 h, exhibits superior catalytic performance and achieves a current density of 10 mA/cm2 at an exceptionally low overpotential of merely 476 mV. This research offers a novel reference for the development of high-performance electrochemical water oxidation catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


