聚苯乙烯颗粒通过IL-33分泌诱导哮喘样th2介导的肺损伤
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
塑料,特别是聚苯乙烯(PS),在世界范围内被广泛使用,特别是在一次性包装中,它通过产生微塑料颗粒而造成环境污染。在此,我们研究了PS微塑料的肺毒性作用,重点是气道炎症和免疫反应。PS微塑料(50 nm至1 μm)暴露更有可能引起严重的肺部炎症反应,特别是较小的颗粒尺寸。PS微塑料仅通过鼻子吸入导致肺部毒性作用,该研究特别关注通过吸入人体接触空气中的微塑料。我们证明了PS微塑料暴露在小鼠中会导致明显的哮喘样症状,包括气道炎症、气道高反应性、支气管上皮粘液细胞增生以及通过IL-33信号通路产生的Th2免疫反应。此外,空间转录组分析表明,上皮细胞在ps诱导的肺损伤中驱动IL-33信号通路和Th2细胞活化。经条件培养基处理的ps刺激的原代上皮细胞在C57BL/6小鼠源性脾细胞中增加Th2免疫应答,包括细胞因子水平和mRNA表达。同时,IL-33抑制剂或地塞米松治疗可有效调节PS暴露引起的th2介导的肺部炎症。这些发现增强了我们对呼吸系统中微塑料暴露的毒理学影响的理解,并有助于制定潜在的缓解策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
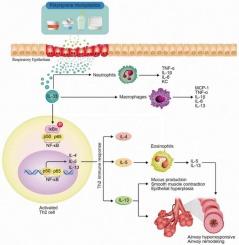
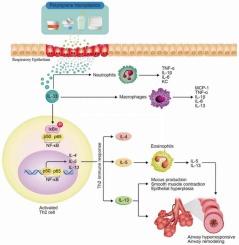
Polystyrene particles induces asthma-like Th2-mediated lung injury through IL-33 secretion
Plastics, particularly polystyrene (PS), are extensively used worldwide, especially in disposable packaging, which contributes to environmental pollution by generating microplastic particles. Herein, we investigated the pulmonary toxic effects of PS microplastics, focusing on airway inflammation and immune response. PS microplastic (50 nm to 1 μm) exposure was more likely to cause a severe pulmonary inflammatory response, particularly with smaller particle sizes. PS microplastic nose-only inhalation led to pulmonary toxic effects, which is specifically focusing on airborne microplastic exposure via inhalation in humans. We demonstrated that PS microplastic exposure in mice led to significant asthma-like symptoms, including airway inflammation, airway hyperresponsiveness, bronchial epithelial mucus cell hyperplasia, and Th2 immune responses through the IL-33 signalling pathway. Additionally, spatial transcriptome analysis indicated that epithelial cells drive the IL-33 signalling pathway and Th2 cell activation within PS-induced lung injury. PS-stimulated primary epithelial cells with the conditioned medium treatment in C57BL/6 mouse-derived splenocytes increased the Th2 immune response, including cytokine levels and mRNA expression. Meanwhile, Th2-mediated lung inflammation induced by PS exposure was effectively regulated by an IL-33 inhibitor or dexamethasone treatment. These findings enhance our understanding of the toxicological implications of microplastic exposure in the respiratory system and assist in developing potential mitigation strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


