zncl2催化4-烷氧羰基-1,2-二氮杂-1,3-二烯与1,3,5-三嗪烷环化制备咪唑烷的非类碳方法
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以zncl2为催化剂,实现了1,2-二氮杂-1,3-二烯(dd)与六氢-1,3,5-三嗪(ht)的形式[2 + 2 + 1]环化反应,提供了具有季碳中心的咪唑烷骨架。因此,一个新的机会,绕过危险的重氮试剂的使用是可能的独特的类碳反应性(C1合成),容易获得和安全的4-烷氧羰基-1,2-重氮-1,3-丁二烯。此外,利用芳香胺、1,2-二氮-1,3-二烯和1,3,5-三嗪合成不同取代的1,3-二氮基咪唑烷,这种非类碳转化可以实现为两步三组分方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
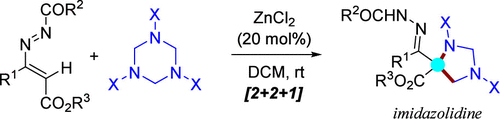
A Noncarbenoid Approach to Imidazolidines via ZnCl2-Catalyzed Annulation of 4-Alkoxycarbonyl-1,2-diaza-1,3-dienes with 1,3,5-Triazinanes
An unprecedented ZnCl2-catalyzed formal [2 + 2 + 1] annulation of 1,2-diaza-1,3-dienes (DDs) with hexahydro-1,3,5-triazines (HTs) has been accomplished, which provides imidazolidine frameworks with quaternary carbon centers. Thus, a new opportunity bypassing the use of hazardous diazo reagents is made possible by a unique carbene-like reactivity (C1 synthon) of readily available and safe 4-alkoxycarbonyl-1,2-diaza-1,3-butadienes. Besides, this noncarbenoid transformation can be implemented into a two-step three-component approach by utilizing the aromatic amine, 1,2-diaza-1,3-dienes and 1,3,5-triazines to synthesize differently substituted 1,3-diaryl imidazolidines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


