揭示肠道影响:急性刚地弓形虫感染可引起雌性C57BL/6小鼠严重的形态学和免疫学变化
IF 1.6
4区 医学
Q3 PARASITOLOGY
引用次数: 0
摘要
弓形虫病是由原生动物刚地弓形虫(弓形虫)引起的一种常见的人畜共患疾病,成人血清阳性率高达60%。虽然通常无症状,但它可以在免疫功能低下的个体中引起严重的并发症。口腔传播是感染的主要途径,导致肠道炎症。本研究评价了刚地弓形虫急性感染雌性C57BL/6小鼠结肠形态定量变化。小鼠分为对照组和感染组,感染后5天实施安乐死收集结肠。组织学分析量化上皮内淋巴细胞、杯状细胞、肥大细胞和胶原纤维,而免疫染色评估神经元和血管活性肠肽(VIP)的表达。感染小鼠表现出上皮内淋巴细胞和髓过氧化物酶活性增加,同时ab -1.0阳性杯状细胞显著减少,表明粘液分泌受损。值得注意的是,纵肌层厚度增加,而粘膜下层和隐窝深度减少。组织病理学检查显示上皮增生、粘膜溃疡和脓肿形成。虽然VIP的表达保持不变,但也观察到肌肠神经元的减少。这些发现表明,急性弓形虫感染引起肠道结构的实质性改变,包括免疫细胞浸润、杯状细胞耗竭和肌肉层重塑。尽管VIP的表达稳定,但肌内神经元的减少提示受感染肠道中存在特异性神经免疫相互作用。总之,本研究强调了弓形虫对结肠形态和功能的深远影响,强调了急性感染期间宿主-寄生虫相互作用的复杂性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
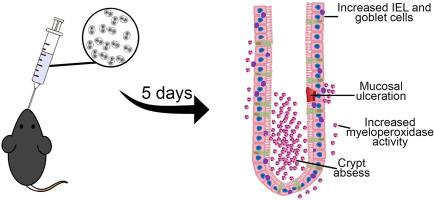
Unmasking the intestinal impact: Acute Toxoplasma gondii infection induces severe morphological and immunological changes in female C57BL/6 mice
Toxoplasmosis, caused by the protozoan Toxoplasma gondii (T. gondii), is a common zoonotic disease with a seropositivity rate of up to 60 % in adults. While often asymptomatic, it can cause severe complications in immunocompromised individuals. Oral transmission is the primary route of infection, leading to intestinal inflammation. This study evaluated morphoquantitative changes in the colons of female C57BL/6 mice acutely infected with T. gondii. Mice were divided into control and infected groups and euthanized five days post-infection for colon collection. Histological analyses quantified intraepithelial lymphocytes, goblet cells, mast cells, and collagen fibres, while immunostaining assessed neurons and vasoactive intestinal peptide (VIP) expression. Infected mice exhibited increased intraepithelial lymphocytes and myeloperoxidase activity, alongside a significant reduction in AB-1.0-positive goblet cells, indicating impaired mucus secretion. Notably, the longitudinal muscle layer showed increased thickness, whereas the submucosal layer and crypt depth were reduced. Histopathological evaluation revealed epithelial hyperplasia, mucosal ulceration, and abscess formation. A decrease in myenteric neurons was also observed, although VIP expression remained unchanged. These findings demonstrate that acute T. gondii infection induces substantial alterations in intestinal structure, including immune cell infiltration, goblet cell depletion, and muscle layer remodelling. The reduction in myenteric neurons, despite stable VIP expression, suggests specific neuroimmune interactions in the infected gut. Collectively, this study highlights the profound impact of T. gondii on colonic morphology and function, underscoring the complexity of host–parasite interactions during acute infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental parasitology
医学-寄生虫学
CiteScore
3.10
自引率
4.80%
发文量
160
审稿时长
3 months
期刊介绍:
Experimental Parasitology emphasizes modern approaches to parasitology, including molecular biology and immunology. The journal features original research papers on the physiological, metabolic, immunologic, biochemical, nutritional, and chemotherapeutic aspects of parasites and host-parasite relationships.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


