人硫转移酶荧光雌激素模拟7-羟基香豆素探针底物的设计
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
磺化是影响雌激素体内平衡的药物代谢反应之一。C-3芳基取代7-羟基香豆素是荧光雌激素模拟物;也就是说,雌激素和7-羟基香豆素的羟基都是由人硫转移酶(SULTs)偶联的。SULTs对7-羟基的磺化降低了7-羟基香豆素的荧光。一系列7-羟基香豆素的磺化是根据这一性质确定的。7-氢香豆素的SULT亚型特异性结合相互作用根据磺化所需的模型最佳排列进行了评估。3-(4-甲氧基苯基)-7-羟基香豆素(11)和3-(4-羟基苯基)-7-羟基香豆素(9)是SULT1E1的选择性底物,而3-(1h -1,2,4-三唑-1-基)-7-羟基香豆素(14)是SULT1A1的选择性底物。其他测试的7-羟基香豆素被两个以上的SULTs磺化。SULT1A1或SULT1C4对大多数7-羟基香豆素的磺化反应遵循Michaelis-Menten动力学,而SULT1E1对几种衍生物的磺化反应则发生底物抑制动力学。衍生物9和11被SULT1E1选择性磺化是由于该酶的长圆柱形活性位点确保在前体反应状态下最佳的7 -羟基位置。与SULT1E相比,SULT1A1和SULT1C4更倾向于较小的衍生物作为底物。雌激素能有效抑制SULT1E1对3,4-二甲基-7-羟基香豆素(4)的磺化作用(IC50小于1µM)。雌激素对SULT1A1和SULT1C4的抑制作用较弱。几种7-羟基香豆素衍生物在SULT1E1和SULT1A1活性位点与雌激素具有共同的结合相互作用模式。荧光7-羟基香豆素可以作为SULTs的探针底物,在药物开发过程中评估其对新化学实体的抑制作用。7-羟基香豆素9或11可作为SULT1E1的选择性探针底物,14可作为SULT1A1的选择性探针底物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
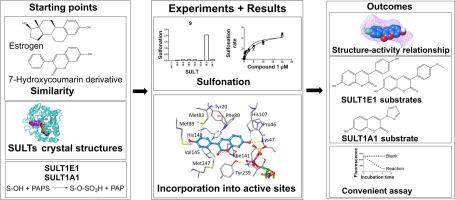
Designing fluorescent estrogen mimetic 7-hydroxycoumarin probe substrates for human sulfotransferase enzymes
Sulfonation is one of drug metabolism reactions affecting homeostasis of estrogens. C-3 aryl substituted 7-hydroxycoumarins are fluorescent estrogen mimetics; i.e., the hydroxyl groups of both estrogens and 7-hydroxycoumarins are conjugated by human sulfotransferases (SULTs). Sulfonation of the 7‑hydroxyl group by SULTs decreases the fluorescence of 7-hydroxycoumarins. Sulfonation of a series of 7-hydroxycoumarins by human SULTs was determined based on this property. SULT subtype-specific binding interactions of 7-hydrocoumarins were assessed against the modelled optimal arrangement needed for sulfonation. 3-(4-Methoxyphenyl)-7-hydroxycoumarin (11) and 3-(4-hydroxyphenyl)-7-hydroxycoumarin (9) were selective substrates for SULT1E1, whereas 3-(1H-1,2,4-triazol-1-yl)-7-hydroxycoumarin (14) was a selective SULT1A1 substrate. Other tested 7-hydroxycoumarin were sulfonated by more than two SULTs. Sulfonation of most 7-hydroxycoumarins by SULT1A1 or SULT1C4 followed Michaelis-Menten kinetics, while substrate inhibition kinetics occurred in sulfonation of several derivatives by SULT1E1. Selective sulfonation of derivatives 9 and 11 by SULT1E1 was due to the enzyme’s long and cylindrical active site that assures optimal 7‑hydroxyl group placement in the precursory reaction state. SULT1A1 and SULT1C4 preferred smaller derivatives as substrates than the SULT1E. Estrogens potently inhibited the sulfonation of 3,4-dimethyl-7-hydroxycoumarin (4) by SULT1E1 (IC50 below 1 µM). SULT1A1 and SULT1C4 were less potently inhibited by the estrogens. Several 7-hydroxycoumarin derivatives share common binding interaction patterns with the estrogens at SULT1E1 and SULT1A1 active sites. Fluorescent 7-hydroxycoumarins could serve as convenient probe substrates for SULTs to evaluate their inhibition by new chemical entities during drug development. 7-Hydroxycoumarins 9 or 11 could be used as selective probe substrates for SULT1E1 and 14 for SULT1A1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


