组氨酸修饰的UiO-66(Zr)纳米颗粒作为5-氟尿嘧啶药物递送系统的有效ph响应载体:一种更有效治疗脑癌的可能途径
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
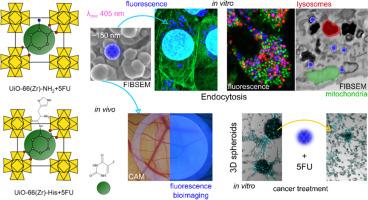
ph反应性药物递送系统的发展对于改善化疗药物在肿瘤微环境中的靶向释放至关重要。在本研究中,研究了UiO-66(Zr)- nh2及其合成后组氨酸(His)修饰形式UiO-66(Zr)-His作为5-氟尿嘧啶(5FU)的潜在载体。他的功能化是通过酰胺键形成实现的,结合效率为62 %。5FU对UiO-66(Zr)- nh2的包封效率为137.1 mg g−1,对UiO-66(Zr)-His的包封效率为45.0 mg g−1,表明表面修饰对载药能力的影响。37 °C下不同pH条件(2.0、5.5和7.4)下的药物释放研究证实了两种材料的pH响应行为。 10 h后最大的药物释放68 % pH = 2,71在pH % = 5.5,和81年 % pH值= 7.4 uio - 66 (Zr) nh₂,而uio - 66 (Zr)——表现出增强的释放在温和的酸性条件(88 % pH = 5.5)。动力学模型显示Weibull和Higuchi模型提供了最佳拟合(R2 >; 0.9),证实了扩散控制释放。研究了制备的材料在体外与真皮成纤维细胞的高生物相容性,以及在体内与鹌鹑绒毛尿囊膜健康组织胚胎模型的高生物相容性。内吞途径被确定为胺化纳米颗粒进入的主要途径,并被His修饰。Western blot和电镜证实,自噬在5FU生物活性前对成纤维细胞有保护作用。最后,在球状体胶质母细胞瘤U87MG(脑癌)细胞的2D和3D模型中,研究了U87MG(脑癌)细胞通过U87MG (Zr)-NH2和UiO-66(Zr)-His转运5FU的生物活性。荧光成像显示了有效的细胞摄取和细胞毒性作用,特别是UiO-66(Zr)-His+5FU,其与溶酶体的共定位增强,与线粒体的串扰增强。U87MG细胞注射UiO-66(Zr)-His+5FU后,乳酸脱氢酶产量显著增加,3D球体形成减少。这些发现突出了UiO-66(Zr)-His作为基于荧光的光诊断和pH反应化疗的有希望的候选者,特别是通过减少其在微环境为微酸性(pH = 5.9-6.9)的脑肿瘤治疗中的大小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Histidine-modified UiO-66(Zr) nanoparticles as an effective pH-responsive carrier for 5-fluorouracil drug delivery system: A possible pathway to more effective brain cancer treatments
The development of pH-responsive drug delivery systems is crucial for improving the targeted release of chemotherapeutics in tumour microenvironments. In this study, UiO-66(Zr)-NH₂ and its post-synthetically histidine (His)-modified form, UiO-66(Zr)-His, were investigated as potential carriers for 5-fluorouracil (5FU). His functionalisation was achieved through amide bond formation, with a binding efficiency of 62 %. The encapsulation efficiency of 5FU reached 137.1 mg g−1 for UiO-66(Zr)-NH₂ and 45.0 mg g−1 for UiO-66(Zr)-His, demonstrating the impact of surface modifications on drug loading capacity. Drug release studies at 37 °C under different pH conditions (2.0, 5.5, and 7.4) confirmed the pH-responsive behaviour of both materials. The maximum drug release after 10 h was 68 % at pH = 2, 71 % at pH = 5.5, and 81 % at pH = 7.4 for UiO-66(Zr)-NH₂, whereas UiO-66(Zr)-His exhibited enhanced release at mildly acidic conditions (88 % at pH = 5.5). Kinetic modelling revealed that the Weibull and Higuchi models provided the best fit (R2 > 0.9), confirming diffusion-controlled release. The high biocompatibility of the prepared materials was investigated in vitro on dermal fibroblasts and in vivo on the preclinical quail embryonic model of the chorioallantoic membrane as healthy tissue. The endocytotic pathway was identified as the main route for the entry of aminated nanoparticles and modified with His. Autophagy confirmed by Western blot and electron microscopy, protected fibroblasts before 5FU bioactivity. Finally, the biological activity of 5FU transported by UiO-66(Zr)-NH2 and UiO-66(Zr)-His was investigated in 2D and 3D models of glioblastoma U87MG (brain cancer) cells in spheroids. Fluorescence imaging revealed efficient cellular uptake and cytotoxic effects, particularly for UiO-66(Zr)-His+5FU, which showed enhanced colocalisation with lysosomes and crosstalk with mitochondria. Administration of UiO-66(Zr)-His+5FU to U87MG cells resulted in a significant increase in lactate dehydrogenase production and reduction in the formation of 3D spheroids. These findings highlight UiO-66(Zr)-His as a promising candidate for fluorescence-based photodiagnostics and pH-responsive chemotherapy, especially by reducing their size in the treatment of brain tumours where the microenvironment is slightly acidic (pH = 5.9–6.9).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


