ε-己内酯深共晶溶剂无溶剂低温开环聚合及其解聚循环
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
研究了氯化胆碱(ChCl)与有机酸之间的深度共晶溶剂(DESs)对ε-己内酯(CL)开环聚合(ROP)的催化性能。这些有机酸充当氢键供体(HBDs),而氯化胆碱充当氢键受体(HBA)。在15种酸中,(-)-樟脑磺酸(CSA)、磷酸二苯酯(DPP)、2,5-二甲基苯磺酸(DSA)和对甲苯磺酸(PTSA)与ChCl以100:1:1 M比可有效聚聚ε-己内酯(PCL),分子量在8000 ~ 10000 g/mol之间,分散性低(Ð)(1.2 ~ 1.5)。采用差示扫描量热法(DSC)研究了ROP动力学。PTSA与ChCl结合反应速度最快,活化能(Ea)最低。此外,利用热重分析(TGA)研究了PCL解聚成CL单体的过程。值得注意的是,磷钨酸(PTA)表现出最高的解聚活性,在160 °C、1 h的真空蒸馏条件下,CL的收率达到100 %。本文章由计算机程序翻译,如有差异,请以英文原文为准。
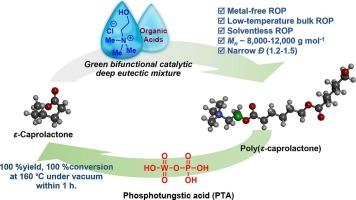
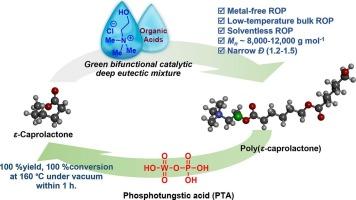
Solventless and low-temperature ring-opening polymerization using deep eutectic solvents of ε-caprolactone and their depolymerization cycles for biodegradable polyesters
The catalytic performance of deep eutectic solvents (DESs) between choline chloride (ChCl) and organic acids was investigated for the ring-opening polymerization (ROP) of ε-caprolactone (CL). These organic acids act as hydrogen bond donors (HBDs), while choline chloride serves as the hydrogen bond acceptor (HBA). Among fifteen acids, (–)-camphorsulfonic acid (CSA), diphenyl phosphate (DPP), 2,5-dimethylbenzenesulfonic acid (DSA), and p-toluenesulfonic acid (PTSA) with ChCl at 100:1:1 M ratio could effectively poly(ε-caprolactone) (PCL) with the molecular weight ranging from 8,000 to 10,000 g/mol and a low dispersity (Ð) (1.2–1.5). The ROP kinetics was powerfully investigated differential scanning calorimetry (DSC) technique. PTSA in combination with ChCl exhibited the fastest reaction and the lowest activation energy (Ea). Additionally, the depolymerization processes of PCL back to its CL monomer was studied by thermogravimetric analysis (TGA). Notably, phosphotungstic acid (PTA) demonstrated the highest depolymerization activity achieving a 100 % yield of CL at 160 °C for 1 h under vacuum distillation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


