成纤维细胞生长因子2通过调节磷酸盐代谢促进基质囊泡介导的人脐带血管周围细胞矿化
IF 2.3
Q1 DENTISTRY, ORAL SURGERY & MEDICINE
引用次数: 0
摘要
目的研究成纤维细胞生长因子2 (FGF2)在促进人脐带血管周围细胞(HUCPVCs)成骨分化和矿化中的作用,并阐明其潜在的分子机制,特别是基质囊泡介导的矿化途径。方法在活化维生素D3、LDN-193189和转化生长因子β1存在的条件下,在成骨条件下培养或不含FGF2的血管内皮细胞。矿化评价采用碱性磷酸酶(ALP)活性测定、茜素红S染色、ALP染色和钙定量。采用选择性FGF受体抑制剂LY2874455和外源性组织非特异性ALP (TNAP)来评价FGF2的作用。利用下一代测序(NGS)、定量聚合酶链反应和免疫荧光染色对基质囊泡介导的矿化和肌成纤维细胞相关标志物进行转录组学分析和表达谱分析。细胞外焦磷酸盐(PPi)用比色法测定。此外,还评估了hucpvc在钛和氧化锆圆盘上的矿化能力。结果fgf2能显著增强ALP活性和矿化,而LY2874455则不能。FGF2上调TNAP和磷酸转运蛋白1,同时抑制外核苷酸焦磷酸酶/磷酸二酯酶1 (ENPP1)和α -平滑肌肌动蛋白的表达。在没有FGF2培养的HUCPVCs中,ENPP1和PPi水平升高与矿化减少相关,通过补充TNAP可以挽救矿化。NGS显示基质囊泡介导的矿化相关基因上调。FGF2在钛和氧化锆圆盘上培养的HUCPVCs有明显的矿化作用。结论sfgf2通过诱导ALP和抑制ENPP1-PPi双重调控增强hucpvc介导的矿化。这些发现支持了FGF2在骨组织工程和种植体表面整合方面的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
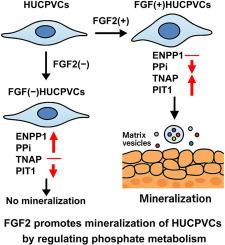
Fibroblast growth factor 2 promotes matrix vesicle-mediated mineralization of human umbilical cord perivascular cells by regulating phosphate metabolism
Objectives
We aimed to investigate the role of fibroblast growth factor 2 (FGF2) in promoting osteogenic differentiation and mineralization of human umbilical cord perivascular cells (HUCPVCs) and to elucidate the underlying molecular mechanisms, particularly the matrix vesicle-mediated mineralization pathway.
Methods
HUCPVCs were cultured under osteogenic conditions with or without FGF2 in the presence of activated vitamin D3, LDN-193189, and transforming growth factor β1. Mineralization was assessed using alkaline phosphatase (ALP) activity assay, Alizarin Red S staining, ALP staining, and calcium quantification. The selective FGF receptor inhibitor LY2874455 and exogenous tissue-non-specific ALP (TNAP) were used to evaluate the effects of FGF2. Transcriptomic analysis and expression profiling of matrix vesicle-mediated mineralization- and myofibroblast-related markers were performed using next-generation sequencing (NGS), quantitative polymerase chain reaction, and immunofluorescence staining. Extracellular pyrophosphate (PPi) was quantified using colorimetric assays. Additionally, the mineralization capacity of HUCPVCs was assessed on titanium and zirconia disks.
Results
FGF2 significantly enhanced ALP activity and mineralization, which were abrogated by LY2874455. FGF2 upregulated TNAP and phosphate transporter 1 while suppressing ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) and alpha-smooth muscle actin expression. In HUCPVCs cultured without FGF2, elevated ENPP1 and PPi levels correlated with reduced mineralization, which was rescued by supplementation with TNAP. NGS revealed upregulation of matrix vesicle-mediated mineralization-related genes. Significant mineralization was observed in HUCPVCs cultured with FGF2 on titanium and zirconia disks.
Conclusions
FGF2 enhances HUCPVC-mediated mineralization via dual regulation: ALP induction and ENPP1–PPi suppression. These findings support the therapeutic potential of FGF2 in bone tissue engineering and implant surface integration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Oral Biosciences
DENTISTRY, ORAL SURGERY & MEDICINE-
CiteScore
4.40
自引率
12.50%
发文量
57
审稿时长
37 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


