巨噬细胞通过tlr诱导的IL-12和IFN-γ从肠上皮细胞龛中协调消除志贺氏菌
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
志贺氏菌属细菌在肠上皮细胞中复制并引起志贺氏菌病,这是一种严重的腹泻疾病,在大多数健康个体中自行消退。在志贺氏菌病期间,中性粒细胞被大量募集到肠道,长期以来被认为是志贺氏菌控制和发病的核心。然而,由于长期缺乏可处理的生理动物模型,志贺氏菌病如何解决仍然知之甚少。在这里,使用我们新开发的Nlrc4 - / - casp11 - / -志贺氏菌病小鼠模型,我们意外地发现中性粒细胞在限制志贺氏菌或疾病发病机制中没有主要作用。相反,我们发现巨噬细胞在宿主控制志贺氏菌中的重要作用。巨噬细胞通过toll样受体(TLRs)对志贺氏菌产生反应,产生IL-12,然后诱导IFN-γ,一种控制肠上皮细胞中志贺氏菌复制所必需的细胞因子。总的来说,我们的发现重塑了我们对志贺氏菌的先天免疫反应的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
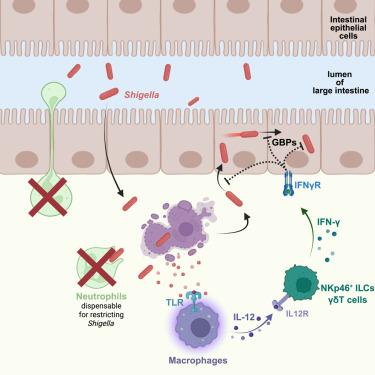
Macrophages orchestrate elimination of Shigella from the intestinal epithelial cell niche via TLR-induced IL-12 and IFN-γ
Bacteria of the genus Shigella replicate in intestinal epithelial cells and cause shigellosis, a severe diarrheal disease that resolves spontaneously in most healthy individuals. During shigellosis, neutrophils are abundantly recruited to the gut and have long been thought to be central to Shigella control and pathogenesis. However, how shigellosis resolves remains poorly understood due to the longstanding lack of a tractable and physiological animal model. Here, using our newly developed Nlrc4–/–Casp11–/– mouse model of shigellosis, we unexpectedly find no major role for neutrophils in limiting Shigella or in disease pathogenesis. Instead, we uncover an essential role for macrophages in the host control of Shigella. Macrophages respond to Shigella via Toll-like receptors (TLRs) to produce IL-12, which then induces IFN-γ, a cytokine that is essential to control Shigella replication in intestinal epithelial cells. Collectively, our findings reshape our understanding of the innate immune response to Shigella.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


