E3连接酶RAD18靶向磷酸化的IRF3以终止IFNB1转录
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
转录因子干扰素调节因子3 (IRF3)启动I型干扰素转录,这是宿主防御所必需的。在这里,我们发现RAD18是一个中心E3泛素连接酶,它选择性地靶向磷酸化的IRF3 (p-IRF3)进行自噬降解。RAD18特异性地促进p-IRF3与IFNB启动子的分离,进而终止其转录活性。在机制上,RAD18结合位于IFNB启动子上的p-IRF3二聚体,并在Lys 193触发p-IRF3的K63多泛素化。泛素化的p-IRF3二聚体因此与IFNB启动子分离,转位出核并经历optn介导的自噬降解。Rad18fl/fl Lysm-cre小鼠体内抗致死性水疱性口炎病毒感染是由于IFNβ过量产生。在h1n1感染的人巨噬细胞或来自活动性系统性红斑狼疮个体的单核细胞中,RAD18蛋白水平与p-IRF3和IFNB1 mRNA水平呈负相关。因此,RAD18作为终止irf3驱动的IFNB1转录的中断,可能是RNA病毒感染或自身免疫性疾病的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
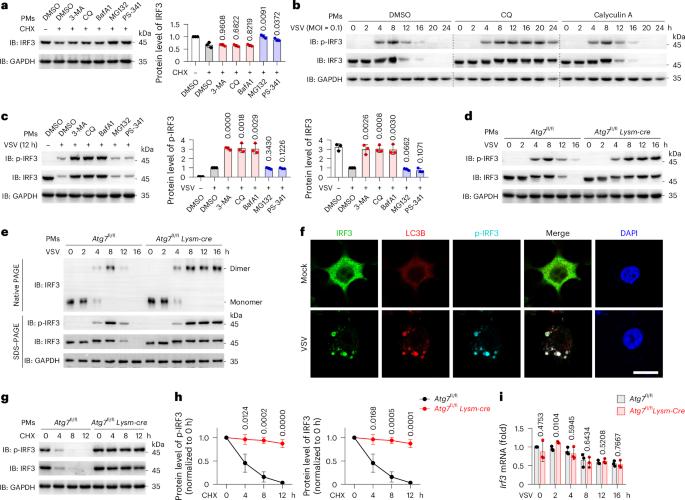
E3 ligase RAD18 targets phosphorylated IRF3 to terminate IFNB1 transcription
The transcription factor interferon regulatory factor 3 (IRF3) initiates type I interferon transcription, which is required for host defense. Here, we identify RAD18 as a central E3 ubiquitin ligase that selectively targets phosphorylated IRF3 (p-IRF3) for autophagic degradation. RAD18 specifically promotes the dissociation of p-IRF3 from the IFNB promoter and in turn terminates its transcriptional activity. Mechanistically, RAD18 binds the p-IRF3 dimer located on the IFNB promoter and triggers K63 polyubiquitylation of p-IRF3 at Lys 193. The ubiquitylated p-IRF3 dimer consequently dissociates from the IFNB promoter, translocates out of the nucleus and undergoes OPTN-mediated autophagic degradation. Rad18fl/fl Lysm-cre mice resist lethal vesicular stomatitis virus infection in vivo due to IFNβ overproduction. In H1N1-infected human macrophages or monocytes from individuals with active systemic lupus erythematosus, RAD18 protein levels negatively correlate with p-IRF3 and IFNB1 mRNA levels. Thus, RAD18 functions as a break to terminate IRF3-driven IFNB1 transcription and may be a potential therapeutic target for RNA virus infection or autoimmune diseases. IRF3 initiates type I IFN transcription, and this is required for host defense. Here, Chen and colleagues show that RAD18 terminates the transcriptional activity of IRF3 and subsequently promotes the autophagic degradation of IRF3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


