L009肽通过调节TNFSF15-VEGF轴作为角膜新生血管的新型抗血管生成剂
IF 5.6
1区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
摘要
角膜新生血管(CNV)是视力损害的主要原因,现有的治疗方案提供有限的疗效。本研究提出了一种新型抗血管生成药物L009,该药物来源于人纤溶酶原的Kringle 5结构域,特别是一种七肽,旨在治疗CNV。在临床前模型中,L009通过显著抑制内皮细胞增殖和迁移,降低血管通透性,维持眼部安全,显示出实质性的疗效。在机制上,L009通过上调肿瘤坏死因子超家族成员15 (TNFSF15),下调血管内皮生长因子受体2 (VEGFR2),增加可溶性VEGFR1的表达来发挥作用。这些作用通过上调紧密连接蛋白ve -钙粘蛋白恢复血管稳态,增强血管稳定性。通过特异性靶向TNFSF15-VEGF轴,L009提供了一种独特的治疗方法,将其与传统的抗vegf疗法区分开来。其抗血管生成和促进血管稳定性的双重作用突出了其作为新一代CNV治疗的潜力。进一步研究其长期疗效和与现有疗法协同作用的潜力是必要的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
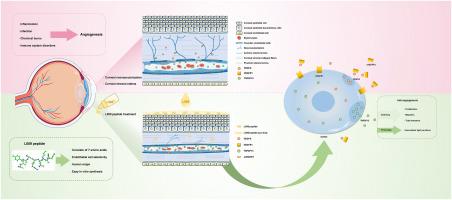
L009 peptide as a novel antiangiogenic agent for corneal neovascularization via regulation of the TNFSF15-VEGF axis
Corneal neovascularization (CNV) is a leading cause of vision impairment, with existing therapeutic options offering limited efficacy. This study presents L009, a novel antiangiogenic agent derived from the Kringle 5 domain of human plasminogen, specifically a heptapeptide, designed to treat CNV. In preclinical models, L009 demonstrated substantial efficacy by markedly inhibiting endothelial cell proliferation and migration, reducing vascular permeability, and maintaining ocular safety. Mechanistically, L009 exerts its effects by upregulating tumor necrosis factor superfamily member 15 (TNFSF15), downregulating vascular endothelial growth factor receptor 2 (VEGFR2), and increasing the expression of soluble VEGFR1. These actions restore vascular homeostasis and enhance vascular stability through the upregulation of the tight junction protein VE-cadherin. By specifically targeting the TNFSF15-VEGF axis, L009 offers a distinctive therapeutic approach, differentiating it from conventional anti-VEGF therapies. Its dual action of anti-angiogenesis and promotion of vascular stability highlights its potential as a next-generation treatment for CNV. Further investigation into its long-term efficacy and potential for synergy with existing therapies is warranted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Ocular Surface
医学-眼科学
CiteScore
11.60
自引率
14.10%
发文量
97
审稿时长
39 days
期刊介绍:
The Ocular Surface, a quarterly, a peer-reviewed journal, is an authoritative resource that integrates and interprets major findings in diverse fields related to the ocular surface, including ophthalmology, optometry, genetics, molecular biology, pharmacology, immunology, infectious disease, and epidemiology. Its critical review articles cover the most current knowledge on medical and surgical management of ocular surface pathology, new understandings of ocular surface physiology, the meaning of recent discoveries on how the ocular surface responds to injury and disease, and updates on drug and device development. The journal also publishes select original research reports and articles describing cutting-edge techniques and technology in the field.
Benefits to authors
We also provide many author benefits, such as free PDFs, a liberal copyright policy, special discounts on Elsevier publications and much more. Please click here for more information on our author services.
Please see our Guide for Authors for information on article submission. If you require any further information or help, please visit our Support Center
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


