SQSTM1/p62在椎间盘退变中的多重作用:细胞应激反应的主要调节因子
IF 2.2
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
椎间盘退变(IDD)是腰椎退行性疾病和慢性腰痛的关键因素。越来越多的证据表明,Sequestosome 1 (SQSTM1/p62)是一种多功能的连接蛋白,通过调节自噬、氧化应激、炎症和程序性细胞死亡,在IDD的发病机制中起着关键作用。本文综述了SQSTM1在IDD中的多方面功能,包括其参与自噬-溶酶体途径,通过Keap1-Nrf2轴进行抗氧化防御,激活NF-κB信号和NLRP3炎性体,以及调节细胞凋亡、焦亡和铁亡。此外,SQSTM1通过上调基质金属蛋白酶和下调其抑制剂来促进细胞外基质降解。鉴于其在椎间盘退变过程中的动态表达,SQSTM1有望成为IDD进展的生物标志物和治疗靶点。针对SQSTM1的潜在策略包括使用自噬诱导剂、炎症途径抑制剂和铁亡/焦亡调节剂。然而,在精确调节SQSTM1活性并将研究结果转化为临床治疗方面仍然存在挑战。利用单细胞RNA测序、蛋白质组学和类器官模型等先进技术的未来研究对于揭示SQSTM1在IDD中的复杂、阶段和细胞特异性作用至关重要。了解这些机制可能为退行性脊柱疾病的有效治疗和改善患者预后开辟新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
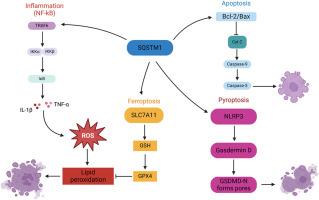
The multifaceted role of SQSTM1/p62 in disc degeneration: A master regulator of cellular stress responses
Intervertebral disc degeneration (IDD) is a key contributor to lumbar degenerative diseases and chronic low back pain. Accumulating evidence indicates that Sequestosome 1 (SQSTM1/p62), a multifunctional adaptor protein, plays a pivotal role in IDD pathogenesis through its regulation of autophagy, oxidative stress, inflammation, and programmed cell death. This review summarizes the multifaceted functions of SQSTM1 in the context of IDD, including its involvement in the autophagy-lysosome pathway, antioxidant defense via the Keap1-Nrf2 axis, activation of the NF-κB signaling and NLRP3 inflammasome, and modulation of apoptosis, pyroptosis, and ferroptosis. Moreover, SQSTM1 contributes to extracellular matrix degradation by upregulating matrix metalloproteinases and downregulating their inhibitors. Given its dynamic expression during disc degeneration, SQSTM1 holds promise as both a biomarker for IDD progression and a therapeutic target. Potential strategies targeting SQSTM1 include the use of autophagy inducers, inflammatory pathway inhibitors, and ferroptosis/pyroptosis modulators. However, challenges remain in precisely modulating SQSTM1 activity and translating findings into clinical therapies. Future research leveraging advanced technologies such as single-cell RNA sequencing, proteomics, and organoid models is essential to unravel the complex, stage- and cell-specific roles of SQSTM1 in IDD. Understanding these mechanisms may open new avenues for effective treatment and improved patient outcomes in degenerative spinal disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemistry and Biophysics Reports
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
191
审稿时长
59 days
期刊介绍:
Open access, online only, peer-reviewed international journal in the Life Sciences, established in 2014 Biochemistry and Biophysics Reports (BB Reports) publishes original research in all aspects of Biochemistry, Biophysics and related areas like Molecular and Cell Biology. BB Reports welcomes solid though more preliminary, descriptive and small scale results if they have the potential to stimulate and/or contribute to future research, leading to new insights or hypothesis. Primary criteria for acceptance is that the work is original, scientifically and technically sound and provides valuable knowledge to life sciences research. We strongly believe all results deserve to be published and documented for the advancement of science. BB Reports specifically appreciates receiving reports on: Negative results, Replication studies, Reanalysis of previous datasets.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


