光触发硫代吡啶卟啉环糊精染料体外灭活氧化孢子镰刀菌
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-08-22
DOI:10.1016/j.jphotochem.2025.116719
引用次数: 0
摘要
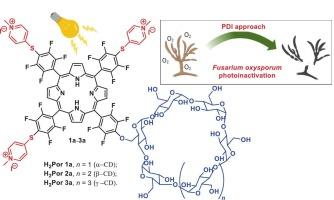
镰刀菌属由广泛分布于土壤和植物中的丝状真菌组成,对包括植物、动物和人类在内的各种生物构成威胁。传统的杀菌剂具有局限性,导致人们对微生物的光动力失活(PDI)等可持续控制方法越来越感兴趣。本研究评估了中位取代卟啉衍生物(H2Pors 1a-3a)的抗真菌光动力活性,每个结构由三个硫代吡啶基团和一个环糊精基团(α-, β-或γ-CD)组成。制备了这些水溶性卟啉环糊精衍生物,并对其在尖孢镰刀菌上进行了实验。在25 mW.cm−2的白光照射下(光剂量为90 J.cm−2),在1.0、2.5和5.0 μM浓度下进行60 min的光失活实验。在最高测试浓度下,所有分子都观察到真菌失活。H2Por 3a表现出最强的PDI效应,在2.5和5.0 μM时实现分生孢子的完全失活(5.0 log10活力降低),在1.0 μM时实现部分失活(~ 3.0 log10活力降低)。H2Por 1a在最高浓度下的效果与H2Por 3a相似,但在最低浓度下效果较差。在最高浓度为5.0 μM时,H2Por 2a可使真菌部分失活(活性降低2 log10)。这种较低的抗真菌活性归因于β-CD在水中形成聚集体。这些结果表明,h2prs 1a和3a是有前途的药物,用于氧化孢子分生孢子的光失活。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In vitro eradication of Fusarium oxysporium conidia using light-triggered thiopyridinium porphyrin-cyclodextrin dyes
The genus Fusarium comprises filamentous fungi widely distributed in soil and plants, representing threats to various organisms, including plants, animals, and humans. Conventional fungicides have limitations, leading to a growing interest in sustainable control methods such as photodynamic inactivation (PDI) of microorganisms. This study evaluates the antifungal photodynamic activity of meso-substituted porphyrin derivatives (H2Pors 1a–3a), which each structure is composed by three thiopyridinium groups and one cyclodextrin group (α-, β-, or γ-CD). These water-soluble porphyrin-cyclodextrin derivatives were prepared and tested on Fusarium oxysporium conidia. Photoinactivation tests were conducted at different concentrations (1.0, 2.5, and 5.0 μM) under white light exposure at an irradiance of 25 mW.cm−2 for 60 min (light dose of 90 J.cm−2). Fungal inactivation was observed for all molecules at the highest tested concentration. H2Por 3a demonstrated the strongest PDI effect, achieving complete inactivation of the conidia (5.0 log10 viability reduction) at 2.5 and 5.0 μM and partially at 1.0 μM (∼3.0 log10 viability reduction). H2Por 1a showed similar results to H2Por 3a at the highest concentration, but was less effective at the lowest concentration. H2Por 2a showed partial inactivation of the fungi at the highest tested concentration of 5.0 μM (2 log10 viability reduction). This lower antifungal activity is attributed to the β-CD forming aggregates in water. These findings suggest that H2Pors 1a and 3a are promising drugs for the photoinactivation of F. oxysporium conidia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


