以NiFe/Al2O3为催化剂,在碱性条件下用废咖啡渣制氢
Q1 Environmental Science
引用次数: 0
摘要
本研究探索了一种利用NiFe/Al2O3作为催化剂,通过碱性热处理(ATT)从废咖啡渣(SCG)中可持续制氢的创新方法。通过一系列单因素实验考察了ATT关键参数对氢气和甲烷产率的影响。随后,使用Box-Behnken设计(BBD)模型实现了工艺优化。最佳操作条件为:600℃,氢氧化钠浓度(SHC)为33%,氢氧化钠溶液体积质量比(RSSC)为4 mL/g,催化剂与生物质(RCB)比为0.23:1 g/g。在此条件下,氢气和甲烷的产率分别为19.27和2.31 mmol/g,并进行了实验验证。利用brunauer - emmet - teller (BET)、x射线衍射(XRD)、程序升温还原(H2-TPR)和场发射扫描电镜(FE-SEM)对新鲜和废NiFe/Al2O3进行了表征。naoh溶液处理后的SCG的XRD结果表明,SCG由晶体结构向非晶结构转变,有利于烃链的断裂和氢的释放。此外,铁基催化剂中镍的存在促进了烃链的裂解,从而促进了制氢。通过将镍掺入催化剂和NaOH溶液处理来增强制氢的阐明,为从SCG中制氢的可行途径提供了有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
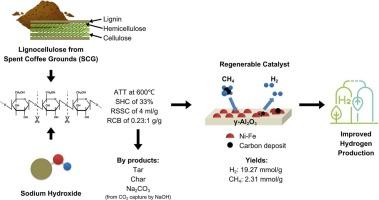
Hydrogen production from spent coffee grounds in alkaline conditions using NiFe/Al2O3 as a catalyst
This study explores an innovative approach to sustainable hydrogen production from spent coffee grounds (SCG) via alkaline thermal treatment (ATT) utilizing Ni![]() Fe/Al2O3 as a catalyst. A series of single-factor experiments were conducted to examine the impact of key ATT parameters on hydrogen and methane yields. Subsequently, process optimization was achieved using a Box-Behnken Design (BBD) model. The optimal operational conditions were 600 °C, a sodium hydroxide concentration (SHC) of 33 %, a sodium hydroxide solution volume to SCG mass (RSSC) ratio of 4 mL/g, and a catalyst to biomass (RCB) ratio of 0.23:1 g/g. Hydrogen and methane yields of 19.27 and 2.31 mmol/g were obtained upon this condition and were experimentally confirmed. The fresh and spent Ni
Fe/Al2O3 as a catalyst. A series of single-factor experiments were conducted to examine the impact of key ATT parameters on hydrogen and methane yields. Subsequently, process optimization was achieved using a Box-Behnken Design (BBD) model. The optimal operational conditions were 600 °C, a sodium hydroxide concentration (SHC) of 33 %, a sodium hydroxide solution volume to SCG mass (RSSC) ratio of 4 mL/g, and a catalyst to biomass (RCB) ratio of 0.23:1 g/g. Hydrogen and methane yields of 19.27 and 2.31 mmol/g were obtained upon this condition and were experimentally confirmed. The fresh and spent Ni![]() Fe/Al2O3 were characterized using Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), temperature programmed reduction (H2-TPR), and field emission scanning electron microscopy (FE-SEM). The XRD results of NaOH-solution treated SCG revealed a transition from a crystalline structure to a more amorphous structure, which facilitates the scission of hydrocarbon chains and enhances hydrogen release. In addition, the presence of Ni in Fe-based catalysts enhances the cracking of hydrocarbon chain and thus hydrogen production. The elucidation of enhanced hydrogen generation through nickel incorporation into the catalyst and NaOH solution treatment provide valuable insights into a viable route for producing hydrogen from SCG.
Fe/Al2O3 were characterized using Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), temperature programmed reduction (H2-TPR), and field emission scanning electron microscopy (FE-SEM). The XRD results of NaOH-solution treated SCG revealed a transition from a crystalline structure to a more amorphous structure, which facilitates the scission of hydrocarbon chains and enhances hydrogen release. In addition, the presence of Ni in Fe-based catalysts enhances the cracking of hydrocarbon chain and thus hydrogen production. The elucidation of enhanced hydrogen generation through nickel incorporation into the catalyst and NaOH solution treatment provide valuable insights into a viable route for producing hydrogen from SCG.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioresource Technology Reports
Environmental Science-Environmental Engineering
CiteScore
7.20
自引率
0.00%
发文量
390
审稿时长
28 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


