杂化聚氨酯丙烯酸酯薄膜通过巯基点击和溶胶-凝胶反应:单体依赖的结构-性能关系
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究介绍了一种将巯基化学与溶胶-凝胶工艺相结合来增强紫外光固化聚氨酯丙烯酸酯(PUA)涂料的新策略。以双缩脲型六亚乙烯二异氰酸酯(HDI)和季戊四醇(PENTA)为核心,用三种结构不同的丙烯酸单体:2-甲基丙烯酸羟乙酯(HEMA)、2-丙烯酸羟乙酯(HEA)和2-甲基丙烯酸羟丙酯(HPMA)进行端盖,合成了杂化聚氨酯丙烯酸酯(HPUA)薄膜。随后,在紫外照射下,通过巯基点击反应将(3-巯基)三甲氧基硅烷(MPTMS)接枝到丙烯酸酯端聚合物上,引入烷氧基硅烷基团进行溶胶-凝胶杂化。目的是研究单体结构如何影响有机-无机网络的发展以及由此产生的热、机械和表面性能。FTIR光谱反褶积证实了氢键和交联的增强,特别是在基于hpma的体系中。DSC和TGA结果表明,HPMA-HPUA表现出最高的玻璃化转变温度(Tg从- 13.52°C到90.0°C)和热稳定性(T50从380°C到457°C),这归因于与二氧化硅网络的界面相容性改善。表面分析表明,溶胶-凝胶改性后的硬度和疏水性增加,接触角上升到130°。该研究为依赖单体的杂化建立了清晰的结构-性能关系框架,并提出了一种可扩展的方法,用于设计具有可定制的热性能和机械性能的高性能uv固化涂层。本文章由计算机程序翻译,如有差异,请以英文原文为准。
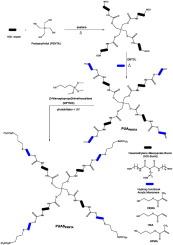
Hybrid polyurethane acrylate films via thiol–ene click and sol–gel reactions: Monomer-dependent structure–property relationships
This study introduces a novel strategy for enhancing UV-curable polyurethane acrylate (PUA) coatings by integrating thiol–ene click chemistry with sol–gel processing. Hybrid polyurethane acrylate (HPUA) films were synthesized using biuret-type hexamethylene diisocyanate (HDI) and a pentaerythritol (PENTA) core, followed by end-capping with three structurally distinct acrylate monomers: 2-hydroxyethyl methacrylate (HEMA), 2-hydroxyethyl acrylate (HEA), and 2-hydroxypropyl methacrylate (HPMA). Subsequently, (3-mercaptopropyl)trimethoxysilane (MPTMS) was grafted onto the acrylate-terminated polymers via a thiol–ene click reaction under UV irradiation to introduce alkoxysilane groups for sol–gel hybridization. The objective was to investigate how monomer architecture influences the development of organic–inorganic networks and the resulting thermal, mechanical, and surface properties. FTIR spectral deconvolution confirmed enhanced hydrogen bonding and crosslinking, especially in HPMA-based systems. DSC and TGA results showed that HPMA–HPUA exhibited the highest glass transition temperature (Tg from −13.52 °C to 90.0 °C) and thermal stability (T50 from 380 °C to 457 °C), attributed to improved interfacial compatibility with the silica network. Surface analyses revealed increased hardness and hydrophobicity after sol–gel modification, with contact angles rising up to 130°. This study establishes a clear structure–property relationship framework for monomer-dependent hybridization and presents a scalable approach for designing high-performance, UV-curable coatings with customizable thermal and mechanical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


