以铜冶炼浮选渣为原料合成光催化析氢TiO2/铜基氧化物光催化复合材料
IF 7.9
3区 材料科学
Q1 GREEN & SUSTAINABLE SCIENCE & TECHNOLOGY
引用次数: 0
摘要
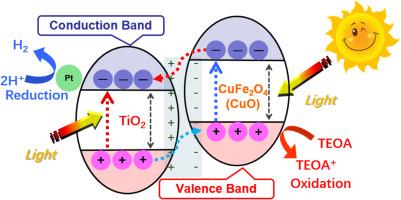
纯TiO2及其复合材料可通过两步光催化还原制氢:光催化还原Pt (IV)和随后的H2析出。以乌兹别克斯坦产铜浮选渣为原料,合成了用于制氢的光催化剂。通过煅烧(Cal900Air5h),提高了渣中CuFe2O4和Fe3O4两种主要相的结晶度。随后,通过原位水热反应(TiO2- 2-5 %Cal900Air5h_HT)合成了Cal900Air5h。TiO2-5 %Cal900Air5h_HT催化H2的生成,是纯TiO2的2.6倍。此外,最有趣的结果是,TiO2-5 %Cal900Air5h_HT的h2生成活性不仅高于纯TiO2和物理混合的复合材料(TiO2-5 %Cal900Air5h_PM),而且也高于试剂基水热制备的TiO2复合材料(TiO2-5 %CuFe2O4, Fe3O4, CuO_HT)。这表明Cal900Air5h中的Si、Ca和Al等非晶态相本身就是绝缘体,可能间接参与了光催化反应。光催化析氢的可能机制如下:通过各种技术测定的能带结构表明,煅烧材料中TiO2与CuFe2O4之间主要形成II型异质结,而TiO2与CuO之间较少形成异质结。因此,当复合材料暴露于光下时,在煅烧渣中的TiO2和CuFe2O4(略CuO)两相中都有电子被激发,然后被激发的电子通过异质结从CuFe2O4和CuO转移到TiO2上。电子聚集在TiO2侧,光催化将H2O分子还原成H2在Pt(0)纳米粒子表面作为助催化剂。本研究独特地探索了回收铜渣作为一种经济、可持续的光催化剂,用于析氢以实现碳中和。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis of TiO2/copper-based oxide photocatalytic composites from copper smelting flotation slag for photocatalytic H2 evolution
Pure TiO2 and its composites can be used for hydrogen production by a two-step photocatalytic reduction: photocatalytic reduction of Pt (IV) and subsequent H2 evolution. Here, photocatalysts were synthesized from copper smelting flotation slags from Uzbekistan for hydrogen production. The crystallinity of the two main phases of the slag, CuFe2O4 and Fe3O4, was increased by calcinating the slag (Cal900Air5h). Subsequently, the Cal900Air5h was composited with TiO2 by in-situ hydrothermal reaction (TiO2-5 %Cal900Air5h_HT). The TiO2-5 %Cal900Air5h_HT catalyzed the production of a significant amount of H2, 2.6 times more than pure TiO2. Moreover, the most interesting result is that the TiO2-5 %Cal900Air5h_HT showed H2-generating activity not only higher than pure TiO2 and physically mixed composite (TiO2-5 %Cal900Air5h_PM), but also higher than the reagent-based hydrothermally fabricated TiO2 composite (TiO2-5 %CuFe2O4, Fe3O4, CuO_HT). This suggested other amorphous phases including Si, Ca, and Al in Cal900Air5h, which are insulators by themselves, might contribute in an indirect way to the photocatalytic reactions. A possible mechanism of the photocatalytic H2 evolution is as follows: The band structure determined using various techniques indicated that a Type II heterojunction formed mainly between TiO2 and CuFe2O4 and less between TiO2 and CuO in the calcined material. Therefore, when the composite was exposed to light, electrons were excited in both phases of TiO2 and CuFe2O4 (slightly CuO) in the calcined slag, and then the excited electrons were transferred from CuFe2O4 and CuO to TiO2 through the heterojunction. The electrons were accumulated on the TiO2 side, and the photocatalytic reduction of H2O molecules into H2 on the surfaces of Pt (0) nanoparticles serving as a cocatalyst. This study uniquely explored the recycling of copper slag as an econtomical and sustainable photocatalyst for hydrogen evolution to achieve carbon neutrality.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Sustainability
Multiple-
CiteScore
5.80
自引率
6.40%
发文量
174
审稿时长
32 days
期刊介绍:
Materials Today Sustainability is a multi-disciplinary journal covering all aspects of sustainability through materials science.
With a rapidly increasing population with growing demands, materials science has emerged as a critical discipline toward protecting of the environment and ensuring the long term survival of future generations.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


