大戟膜A−G:大戟根中的高级二萜
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对大戟根进行植物化学研究,得到10个高萜类化合物,包括7个未描述的化合物(1-7)和3个类似物(8 - 10),并通过化学相关得到4个衍生物(7a和9a - 9c)。值得注意的是,化合物1是一种罕见的二萜。利用光谱学、ECD、化学和单晶x射线衍射手段对其结构进行了全面表征。细胞毒性筛选表明,7 (7a)的氧化衍生物对非小细胞肺癌(NSCLC)细胞株HCC827表现出最有效的活性,与阳性对照顺铂相当。机制研究表明,7a可将细胞周期阻滞在G1/S期,诱导细胞凋亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
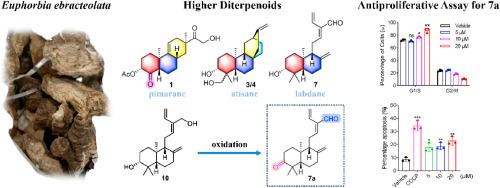
Euphebranes A−G: higher diterpenoids from the radix of Euphorbia ebracteolata
Phytochemical investigation of roots of Euphorbia ebracteolata Hayata led to ten higher diterpenoids including seven undescribed ones (1–7) and three analogues (8−10), accompanied by four derivatives (7a and 9a−9c) obtained via chemical correlation. Notably, compound 1 represents a rare dinor-ent-isopimarane diterpenoid. Their structures were fully characterized using a combination of spectroscopic, ECD, chemical, and single-crystal X-ray diffraction means. Cytotoxicity screening suggested that the oxidized derivative of 7 (7a) displayed the most potent activity against non-small-cell lung cancer (NSCLC) cell line HCC827, comparable to the positive control, cisplatin. Mechanistic study revealed that 7a could arrest cell cycle at G1/S phase and induce apoptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


