用于环境研究的高效干细胞衍生小鼠胚胎模型
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
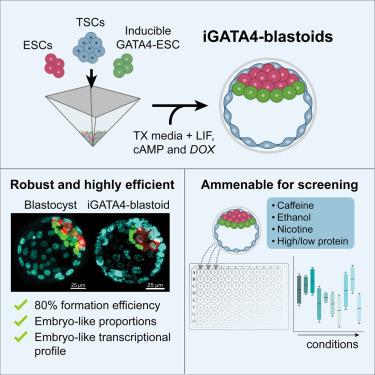
囊胚是模仿天然囊胚的干细胞衍生结构,包含所有三种谱系:滋养外胚层、外胚层和原始内胚层。然而,目前的方法经常产生不完整的结构,不能空化或形成适当的原始内胚层。为了克服这些限制,我们开发了一种模块化方法,通过聚集三种小鼠干细胞类型:胚胎干细胞(ESCs),诱导GATA4表达的ESCs (iG4-ESCs)和滋养细胞干细胞(TSCs)。这种方法产生空泡囊胚样结构,称为ig4囊胚,效率约为80%。单细胞RNA测序证实它们与成熟小鼠囊胚非常相似。值得注意的是,在没有FGF4的情况下培养ig4囊胚增强了侵袭性壁滋养外胚层的特异性,大约12%的结构在体外经历了类似植入后的形态发生。使用该模型,我们发现咖啡因、酒精、尼古丁和氨基酸的变化对ig4囊胚和天然胚胎的影响相似,强调了它们作为研究不同环境因素对胚胎发生影响的强大模型的效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficient stem cell-derived mouse embryo models for environmental studies
Blastoids are stem cell-derived structures that mimic natural blastocysts by incorporating all three lineages: trophectoderm, epiblast, and primitive endoderm. However, current methods often yield incomplete structures that fail to cavitate or to form a proper primitive endoderm. To overcome these limitations, we develop a modular approach by aggregating three murine stem cell types: embryonic stem cells (ESCs), ESCs with inducible GATA4 expression (iG4-ESCs), and trophoblast stem cells (TSCs). This method yields cavitated blastocyst-like structures—termed iG4-blastoids—with approximately 80% efficiency. Single-cell RNA sequencing confirms their close resemblance to mature mouse blastocysts. Notably, culturing iG4-blastoids without FGF4 enhances specification of the invasive mural trophectoderm, and approximately 12% of structures undergo post-implantation-like morphogenesis in vitro. Using this model, we show that caffeine, alcohol, nicotine, and amino acid variations affect iG4-blastoids and natural embryos similarly, underscoring their utility as a robust model for investigating the impact of diverse environmental factors on embryogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


