慢性DBP暴露可能通过干扰HPO轴导致雌性小鼠生育能力降低
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
本研究采用食物污染法,模拟人类长期环境暴露情景,研究了低剂量邻苯二甲酸二丁酯(DBP)暴露对昆明雌性小鼠生殖系统的多代影响(F0-F2)。结果表明,各代之间存在明显的生殖功能障碍,表现为发情间隔缩短(P < 0.05),发情持续时间延长(P < 0.05)。妊娠数逐渐减少,F1-F2代显著减少(P < 0.01)。此外,F2后代性别比例失衡。组织病理学分析显示卵巢的累积损伤是代际的,其特征是闭锁卵泡增加,颗粒细胞紊乱,子宫腺体结构恶化。蛋白质组学分析确定了代特异性途径改变:与对照组相比,dbp暴露组雌激素信号和氧化还原酶活性途径富集;而代际比较揭示了甲状腺激素合成、细胞连接途径和活性氧介导的致癌途径的差异调节。分子研究表明,DBP通过抑制类固醇基因(Cyp17a1, Hsd3b1)破坏下丘脑-垂体-卵巢(HPO)轴功能,导致血清促卵泡激素(FSH)和黄体生成素(LH)水平升高(P < 0.05)。这些研究结果共同表明,慢性低剂量DBP暴露通过内分泌干扰和氧化应激机制诱导跨代生殖损伤,并通过HPO轴失调逐步影响卵巢功能和生育能力。该研究为多代生殖风险与环境邻苯二甲酸盐暴露相关提供了重要证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
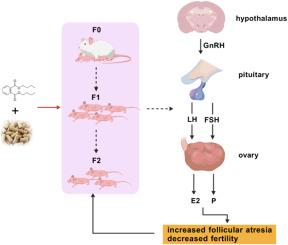
Chronic DBP exposure may cause reduced fertility in female mice by interfering with the HPO axis
In this study, we investigated the multigenerational effects of low-dose dibutyl phthalate (DBP) exposure on the reproductive system of female Kunming mice by simulating a long-term environmental exposure scenario for humans, using a food-contamination method for three consecutive generations (F0-F2). Results demonstrated significant reproductive dysfunction across generations, manifested by shortened diestrus intervals (P < 0.05) and prolonged estrus duration (P < 0.05) in F1-F2 generations. The number of pregnancies progressively declined, with numbers for the F1-F2 generations showing marked reductions (P < 0.01). In addition, the sex ratio of F2 offspring was imbalanced. Histopathological analysis revealed cumulative ovarian damage across generations, characterized by increased atretic follicles, disorganized granulosa cells, and structural deterioration of the uterine glands. Proteomic profiling identified generation-specific pathway alterations: DBP-exposed groups showed enrichment in estrogen signaling and oxidoreductase activity pathways, compared to controls; while intergenerational comparisons revealed differential regulation of thyroid hormone synthesis, cell junction pathways, and reactive oxygen-mediated carcinogenesis pathways. Molecular investigations demonstrated that DBP's disrupted hypothalamic-pituitary-ovarian (HPO) axis function through inhibition of steroidogenic genes (Cyp17a1, Hsd3b1), resulting in elevated serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels (P < 0.05). These findings collectively indicate that chronic low-dose DBP exposure induces transgenerational reproductive impairment through endocrine disruption and oxidative stress mechanisms, progressively compromising ovarian function and fertility across generations via HPO axis dysregulation. The study provides critical evidence for multigenerational reproductive risk associated with environmental phthalate exposure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


