terphenyl phospine TRuPhos:钯催化受阻伯烷基胺芳基化的新型高效配体及其机理澄清
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在钯催化的C-N交叉偶联反应中,Terphenyl phosphines作为强大的支撑配体而上升,导致催化剂负载减少和底物范围扩大。为了扩展terphenyl膦家族,TRuPhos和TSPhos以及它们的2-氨基联苯-ƞ2-C,N钯预催化剂被合成并表征,包括一个单晶衍射配合物。TRuPhos在钯催化许多受阻伯烷基胺的芳化反应中表现出前所未有的效率。机理研究表明:1)氧化加成产物胺加合物的转金属途径A在truphos负载钯催化剂下对受阻伯烷基胺芳化反应不起作用,但对小体积胺芳化反应起作用;2)在氧化加成产物上卤素原子被羟基取代的路径C似乎也不可能;3)氧化加成产物与钠酰胺形成C-N偶联产物的反应表明,途径B可能是Pd/ truphos催化受阻伯烷基胺芳化反应的一个过渡金属途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
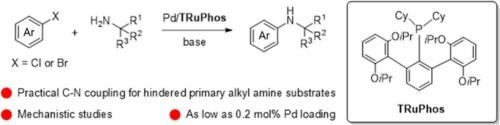
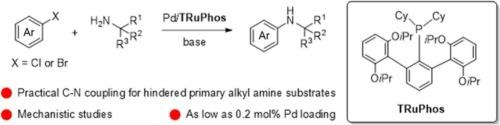
The terphenyl phosphine TRuPhos: a novel and efficient ligand for the palladium-catalyzed arylation of hindered primary alkyl amines with mechanism clarification
Terphenyl phosphines are rising as powerful supporting ligands in the palladium-catalyzed C-N cross-coupling reactions, leading to reduced catalyst loadings and broadened substrate scope. To extend the terphenyl phosphine family, TRuPhos and TSPhos along with their 2-amino-biphenyl-ƞ2-C,N palladium precatalysts have been synthesized and characterized, including one complex by single-crystal diffraction. TRuPhos exhibits unprecedented efficiency in the palladium-catalyzed arylation of many hindered primary alkyl amines. Mechanism studies have clarified that: 1) the transmetalation path A involving amine adducts of oxidative addition product does not work by the TRuPhos-supported palladium catalyst in the arylation of hindered primary alkyl amines, but works for that in small bulk amines; 2) the path C involving the substitution of halogen atoms on oxidative addition products by tBuO groups seems impossible as well; 3) the reaction occurred between oxidative addition products and sodium amides forming C-N coupling products suggests that path B is a possible transmetalation pathway in Pd/TRuPhos-catalyzed arylation of hindered primary alkyl amines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


