线粒体靶向MXene@MnO2-TPP纳米异质结构对骨肉瘤声动力治疗和免疫治疗的协同增强作用
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
线粒体DNA (mtDNA)作为一种内源性危险相关分子模式,广泛激活cGAS-STING通路,从而增强抗肿瘤免疫治疗。然而,低效的mtDNA释放严重限制了其激活下游免疫反应的能力。最近的研究表明,铁下垂可以触发mtDNA从受损的线粒体释放到细胞质中,从而刺激抗肿瘤免疫。因此,精确调节线粒体相关的铁凋亡以促进mtdna依赖性的cGAS-STING激活是一种有希望的增强免疫治疗的策略。在这里,我们设计了一个线粒体靶向MXene@MnO2-TPP肖特基异质结,整合了超声致敏,铁凋亡诱导和免疫激活的协同治疗。该纳米平台不仅直接产生ROS触发肿瘤细胞铁下垂,而且通过mcu依赖的Ca2+内流途径放大铁下垂。此外,它通过释放mtDNA和Mn2+双重激活cGAS-STING通路,刺激I型干扰素的产生,引发全身抗肿瘤免疫。体外和体内研究表明,骨肉瘤小鼠具有强大的肿瘤抑制作用和延长生存期。我们的工作提出了一种创新的“铁- mtdna -免疫治疗”模式,为骨肉瘤的治疗提供了一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
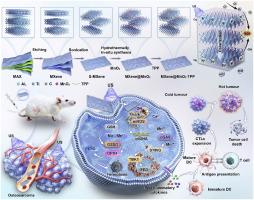
Mitochondria-targeted MXene@MnO2-TPP nanoheterostructures for synergistic enhancement of sonodynamic therapy and immunotherapy in osteosarcoma
Mitochondrial DNA (mtDNA) functions as an endogenous danger-associated molecular pattern that broadly activates the cGAS–STING pathway to potentiate antitumor immunotherapy. However, inefficient mtDNA release severely limits its ability to robustly activate downstream immune responses. Recent studies reveal that ferroptosis can trigger mtDNA release from damaged mitochondria into the cytosol, thereby stimulating antitumor immunity. Thus, precisely modulating mitochondria-associated ferroptosis to promote mtDNA-dependent cGAS–STING activation represents a promising strategy for enhancing immunotherapy. Here, we engineered a mitochondria-targeted MXene@MnO2-TPP Schottky heterojunction that integrates sonosensitization, ferroptosis induction, and immune activation for synergistic therapy. This nanoplatform not only directly generates ROS to trigger tumor cell ferroptosis but also amplifies ferroptosis via an MCU-dependent Ca2+ influx pathway. Furthermore, it dual-activates the cGAS–STING pathway through released mtDNA and Mn2+, stimulating type I interferon production and eliciting systemic antitumor immunity. In vitro and in vivo studies demonstrate robust tumor suppression and prolonged survival in osteosarcoma-bearing mice. Our work proposes an innovative “ferroptosis–mtDNA–immunotherapy” paradigm, offering a promising strategy for osteosarcoma treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


