绿色荧光光谱法测定依那普利的系统建立和综合验证:响应面优化、机理研究及其在制药和生物分析样品中的应用
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-08-22
DOI:10.1016/j.jphotochem.2025.116720
引用次数: 0
摘要
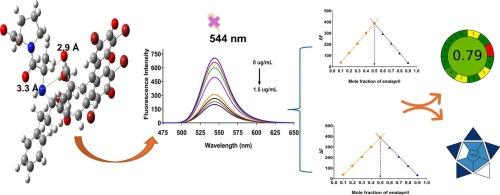
以伊红Y为荧光探针,建立了一种灵敏、选择性、环境友好的抗高血压药物依那普利的荧光测定方法。在酸性pH下,依那普利和伊红Y之间形成离子结合复合物,导致染料的荧光猝灭。通过温度相关的Stern-Volmer分析(298 - 313K)、Job方法、热力学表征和量子力学计算,系统地研究了猝灭机理,揭示了Stern-Volmer常数从8.22 × 105 M−1 (298 K)下降到6.17 × 105 M−1 (313K)的静态相互作用模式。综合热力学分析结果显示出负焓变和负熵变,表明在络合物形成过程中,以强静电相互作用为主的高度放热结合失去了平移自由。Job’s比值证实了1:1的化学计量,摩尔分数为0.5时最大;PM3量子力学计算确定了依那普利的质子化氨基与伊红Y的羧酸基之间的结合位点,证实了1:1的复合物形成。采用响应面法和中心复合设计对pH、缓冲液体积和试剂体积等影响淬火过程的因素进行了优化。通过方差分析识别出显著的建模参数,建立并验证了简化二次元模型,具有良好的预测能力。为了使淬火效率最大化,进行了数值优化。方法在0.05 ~ 1.5 μg/mL范围内具有良好的线性关系,检出限为0.0147 μg/mL。此外,准确度、精密度和稳健性均在可接受范围内。该方法成功地应用于制剂和人血浆样品中依那普利的含量测定,结果令人满意。统计比较报告的高效液相色谱法显示相当的分析性能。此外,分别使用AGREE和BAGI工具对该方法的环境影响和分析实用性进行了评估,表明所提出的荧光光谱技术为依那普利的常规质量控制和治疗药物监测提供了传统HPLC方法的更环保和实用的替代方法,显示了其在制药工业和临床环境中的广泛应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Systematic development and comprehensive validation of a green spectrofluorimetric method for enalapril determination: response surface optimization, mechanistic investigation with application to pharmaceutical and bioanalytical samples
A sensitive, selective, and environmentally friendly spectrofluorimetric method was developed and validated for the determination of enalapril, an antihypertensive drug, using eosin Y as a fluorescent probe. At acidic pH, an ion-association complex is formed between enalapril and eosin Y, resulting in the quenching of the dye's fluorescence. The quenching mechanism was systematically investigated using temperature-dependent Stern-Volmer analysis (298–313 K), Job's method, thermodynamic characterization, and quantum mechanical calculations, revealing a static mode of interaction with Stern-Volmer constants decreasing from 8.22 × 105 M−1 (298 K) to 6.17 × 105 M−1 (313K). Comprehensive thermodynamic analysis provided negative enthalpy and negative entropy changes, indicating highly exothermic binding dominated by strong electrostatic interactions with loss of translational freedom upon complex formation. Job's ratio confirmed 1:1 stoichiometry with maximum at mole fraction 0.5 while PM3 quantum mechanical calculations identified the binding site between the protonated amino group of enalapril and carboxylate group of eosin Y, confirming 1:1 complex formation. Factors affecting the quenching process, including pH, buffer volume, and reagent volume, were optimized using response surface methodology with a central composite design. Significant modeling parameters were identified through ANOVA, and a reduced quadratic model was developed and validated, demonstrating good predictive ability. Numerical optimization was then performed to maximize the quenching efficiency. The proposed method was fully validated according to ICH guidelines, exhibiting excellent linearity in the range of 0.05–1.5 μg/mL, with a limit of detection of 0.0147 μg/mL. Moreover, accuracy, precision, and robustness were within acceptable limits. The developed method was successfully applied for the determination of enalapril in pharmaceutical formulations and spiked human plasma samples with satisfactory results. Statistical comparison to a reported HPLC method revealed comparable analytical performance. Furthermore, the environmental impact and analytical practicality of the method were evaluated using AGREE and BAGI tools, respectively, indicating that the proposed spectrofluorimetric technique offers a greener and practical alternative to traditional HPLC methods for routine quality control and therapeutic drug monitoring of enalapril showcasing its potential for widespread application in the pharmaceutical industry and clinical settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


