水溶液中四硫代酸钠的光化学
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-08-22
DOI:10.1016/j.jphotochem.2025.116721
引用次数: 0
摘要
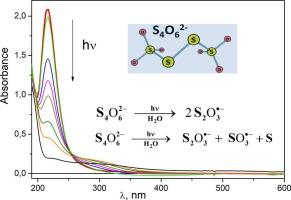
采用固定光解和激光闪光光解两种方法研究了四硫酸阴离子S4O62−在水溶液中的光化学反应。发现S4O62−消失的量子产率随辐照波长和溶解氧的存在从0.05到0.2变化。深层光解导致溶胶的形成。在激光闪光光解实验中检测到S2O3•自由基的吸收。为了定量描述激光闪光光解数据,提出了S4O62−光解的两个通道。第一通道生成两个S2O3•-自由基阴离子;在第二通道中形成S2O3•-和SO3•-自由基阴离子和硫原子。令人惊讶的是,激光闪光光解实验的结果并不依赖于溶解氧的存在。这一事实可以用弱束缚范德华配合物[S4O62−…O2]的形成来解释。在实验条件下,所有的溶解氧分子都是结合在一起的,不能与主自由基阴离子发生反应。在固定光解条件下,氧被光化学释放,并可与其他中间体反应,提高四硫酸盐光解的量子产率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photochemistry of sodium tetrathionate in aqueous solutions
Photochemistry of tetrathionate anion S4O62− in aqueous solutions was studied by means of stationary photolysis and laser flash photolysis. Quantum yield of S4O62− disappearance was found to change from 0.05 to 0.2 depending on the irradiation wavelength and the presence of dissolved oxygen. Deep photolysis leads to the sol formation. S2O3•- radical absorption was detected in the laser flash photolysis experiments. For quantitative description of the laser flash photolysis data, two channels of S4O62− photodissociation were proposed. The first channel yields in formation of two S2O3•- radical anions; in the second channel S2O3•- and SO3•- radical anions and a sulfur atom are formed. Surprisingly, the results of the laser flash photolysis experiments did not depend on the presence of dissolved oxygen. This fact was explained by the formation of the weakly-bound van der Waals complexes [S4O62−…O2]. At the experimental conditions all the dissolved oxygen molecules are bound and cannot react with the primary radical anions. In conditions of stationary photolysis, oxygen is photochemically released and can react with other intermediates, increasing the quantum yield of tetrathionate photodecomposition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


