合成的ZFTA融合物指出了无序蛋白结构域获取作为脑肿瘤发生的机制
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
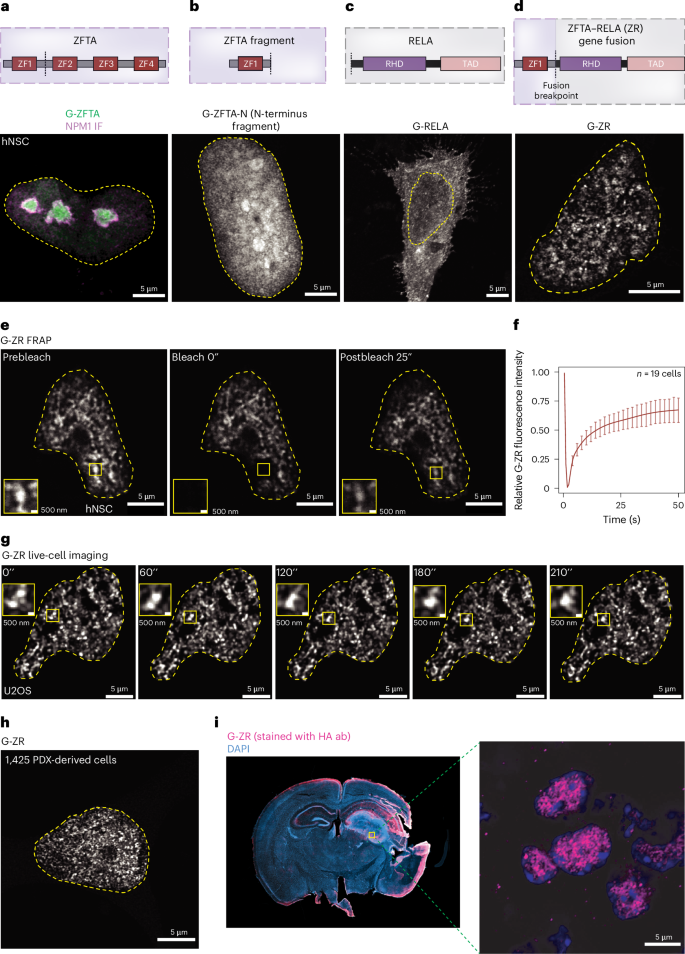
超过95%发生在皮层的室管膜瘤是由锌指易位相关蛋白(ZFTA)基因融合引起的。在这里,使用超分辨率和晶格光片显微镜,我们证明了最常见的融合变异,ZFTA-RELA (ZR),形成癌基因表达和肿瘤发生所需的动态核凝聚物。ZR的诱变研究揭示了RELA中一个关键的内在无序区(IDR)控制凝析物的形成。将凝析物调节的IDR突变引入到ZR中,破坏了其在致癌位点的基因组占用,并抑制了转录效应蛋白(如MED1、BRD4和RNA聚合酶II)的募集。利用核磁共振波谱技术,我们检测了在ZR中发现的临界锌指(ZF1)的dna结合残基,并表征了它们对凝结物形成、基因组结合和癌基因激活的意义。我们合成了ZFTA融合蛋白,将已知凝聚形成蛋白的idr接枝到ZR中。利用来自EWS和FUS的IDRs合成的ZFTA融合癌蛋白恢复了小鼠凝结物形成、癌基因转录和肿瘤起始。这些发现为了解ZR的致癌机制以及IDR获取在脑癌融合癌蛋白中的重要性提供了关键见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Synthetic ZFTA fusions pinpoint disordered protein domain acquisition as a mechanism of brain tumorigenesis
Over 95% of ependymomas that arise in the cortex are driven by a gene fusion involving the zinc finger translocation-associated (ZFTA) protein. Here, using super-resolution and lattice light-sheet microscopy, we demonstrate that the most frequent fusion variant, ZFTA–RELA (ZR), forms dynamic nuclear condensates that are required for oncogene expression and tumorigenesis. Mutagenesis studies of ZR reveal a key intrinsically disordered region (IDR) in RELA that governs condensate formation. Condensate-modulating IDR mutations introduced into ZR impaired its genomic occupancy at oncogenic loci and inhibited the recruitment of transcriptional effector proteins, such as MED1, BRD4 and RNA polymerase II. Using nuclear magnetic resonance spectroscopy, we examined the DNA-binding residues of the critical zinc finger (ZF1) found in ZR and characterized their significance for condensate formation, genomic binding and oncogene activation. We generated synthetic ZFTA fusion proteins where IDRs from known condensate-forming proteins were grafted into ZR. Synthetic ZFTA fusion oncoproteins utilizing IDRs from EWS and FUS restored condensate formation, oncogene transcription and tumour initiation in mice. These findings provide key insights into the oncogenic mechanism of ZR and the importance of IDR acquisition in fusion oncoproteins in brain cancer. Arabzade, Shirnekhi, Varadharajan, Ippagunta and colleagues show that the zinc finger translocation-associated (ZFTA) fusion oncoproteins gain intrinsically disordered regions, inducing nuclear condensate formation and oncogenic activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


