同型半胱甘酸盐通过重塑蛋氨酸代谢和n -同型半胱氨酸化来控制炎症
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
炎症及其代谢网络相互作用产生具有翻译意义的新型调控分子。在这里,我们鉴定了产生同型半胱氨酸的免疫代谢串,同型半胱氨酸和s -腺苷- l-同型半胱氨酸水解酶(AHCY)催化的衣康酸内聚形成的代谢物。同半胱甘酸在炎症期间增加152倍,并表现出抗炎作用。机制上,同型半胱甘酸结合促炎蛋白蛋氨酸- trna合成酶(MARS)的D312残基,抑制其功能,重塑蛋氨酸代谢,反馈抑制n -同型半胱氨酸化途径的早期激活。这种代谢开关促进NLR家族含pyrin结构域3 (NLRP3)泛素化来控制炎症。高胱甘肽酸在脓毒症、高脂肪饮食引起的炎症和结肠炎模型中显示出治疗效果。通过烟酰胺腺嘌呤二核苷酸(NAD+)依赖的AHCY激活(使用烟酰胺核苷和丙酮酸)促进内源性高胱甘酸合成,也通过靶向MARS/ nlrp3 - n -同型半胱氨酸级联抑制炎症。本研究确立了同半胱甘肽酸作为一种抗炎代谢物,通过重编程蛋白质修饰开关作为一种体内平衡调节剂,引入代谢时间调节和临床策略来管理炎症并发症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
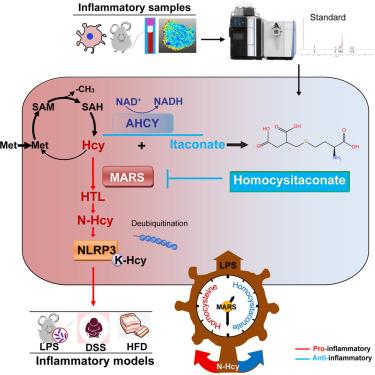
Homocysitaconate controls inflammation through reshaping methionine metabolism and N-homocysteinylation
Inflammation and its metabolic-network interactions generate novel regulatory molecules with translational implications. Here, we identify the immunometabolic crosstalk that generates homocysitaconate, a metabolite formed by homocysteine and itaconate adduction catalyzed by S-adenosyl-L-homocysteine hydrolase (AHCY). Homocysitaconate increases 152-fold during inflammation and exhibits anti-inflammatory effects. Mechanistically, homocysitaconate binds to the D312 residue of the pro-inflammatory protein methionyl-tRNA synthetase (MARS), inhibiting its function and reshaping methionine metabolism to feedback-brake the early activation of the N-homocysteinylation pathway. This metabolic switch facilitates NLR family pyrin domain-containing 3 (NLRP3) ubiquitination to control inflammation. Homocysitaconate demonstrates therapeutic effects in sepsis, high-fat-diet-induced inflammation, and colitis models. Boosting endogenous homocysitaconate synthesis through nicotinamide adenine dinucleotide (NAD+)-dependent AHCY activation (using nicotinamide riboside and pyruvate) also inhibits inflammation by targeting the MARS/NLRP3-N-homocysteinylation cascade. This study establishes homocysitaconate as an anti-inflammatory metabolite that serves as a homeostatic governor by reprogramming protein modification switches, introducing both metabolic timing regulation and clinical strategies to manage inflammatory complications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


